Abstract
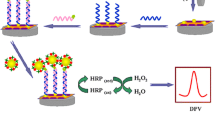
A voltammetric biosensor for lead(II) (Pb2+) is described that is based on signal amplification by using an ion-dependent split DNAzyme and template-free DNA extension reaction. The Pb2+-dependent split DNAzyme was assembled on gold nanoparticles (Au@Fe3O4), and this nanoprobe then was exposed to Pb2+ which causes the split-off of DNAzymes to release primers containing 3′-OH groups (S1 and S2). The template-free DNA extension reaction triggers the generation of long ssDNA nanotails, which then can bind the free redox probe N,N′-bis(2-(trimethylammonium iodide)propylene)perylene-3,4,9,10-tetracarboxyldiimide (PDA+) via electrostatic adsorption. Hence, the concentration of PDA+ in solution is reduced. Therefore, less free PDA+ can be immobilized on a glassy carbon electrode modified with electrodeposited gold nanoparticles (depAu) to produce an electrochemical signal, typically measured at ∼0.38 V (vs. SCE) for quantitation of Pb2+. The use of a Pb2+-dependent split DNAzyme avoids the usage of a proteinic enzyme. It also increases the sensitivity of the sensor which has a lower detection limit of 30 pM of Pb2+.

Novel electrochemical biosensor based on the amplification of ion-dependent split DNAzyme and template-free DNA extension reaction for trace detection of Pb2+.





Similar content being viewed by others
References
Sun J, Luo L (2018) Subcellular distribution and chemical forms of Pb in corn: strategies underlying tolerance in Pb stress. J Agric Food Chem 66(26):6675–6682
Zhu H, Tan X, Tan L, Zhang H, Liu H, Fang M, Wang X (2018) Magnetic porous polymers prepared via high internal phase emulsions for efficient removal of Pb2+ and Cd2+. ACS Sustain Chem Eng 6(4):5206–5213
Tang DP, Xia BY, Tang Y, Zhang J, Zhou Q (2019) Metal-ion-induced DNAzyme on magnetic beads for detection of lead (II) by using rolling circle amplification, glucose oxidase, and readout of pH changes. Microchim Acta 186(5):318–326
Islam E, Liu D, Li T, Yang X, Jin X, Khan MA, Mahmood Q, Hayat Y, Imtiaz M (2011) Effect of Pb toxicity on the growth and physiology of two ecotypes of Elsholtzia argyi and its alleviation by Zn. Environ Toxicol 26(4):403–416
Lo W, Chua H, Lam KH, Bi SP (1999) A comparative investigation on the biosorption of lead by filamentous fungal biomass. Chemosphere 39(15):2723–2736
Huang MR, Ding YB, Li XG (2014) Combinatorial screening of potentiometric Pb (II) sensors from polysulfoaminoanthraquinone solid ionophore. ACS Comb Sci 16(3):128–138
Zhu CH, Zhu WY, Xu L, Zhou XM (2019) A label-free electrochemical aptasensor based on magnetic biocomposites with Pb2+-dependent DNAzyme for the detection of thrombin. Anal Chim Acta 1047:21–27
Shi Y, Wang HY, Jiang XX, Sun B, Song B, Su YY, He Y (2016) Ultrasensitive, specific, recyclable, and reproducible detection of lead ions in real systems through a polyadenine-assisted, surface-enhanced Raman scattering silicon chip. Anal Chem 88(7):3723–3729
Deng DD, Yang H, Liu C, Zhao K, Li JG, Deng AP (2019) Ultrasensitive detection of diclofenac in water samples by a novel surface-enhanced Raman scattering (SERS)-based immunochromatographic assay using AgMBA@ SiO2-Ab as immunoprobe. Sensor Actuat B-Chem 283:563–570
Shi XH, Gu W, Peng WD, Li BY, Chen NN, Zhao K, Xian YZ (2014) Sensitive Pb2+ probe based on the fluorescence quenching by graphene oxide and enhancement of the leaching of gold nanoparticles. ACS Appl Mater Inter 6(4):2568–2575
Annadhasan M, Muthukumarasamyvel T, Sankar Babu VR, Rajendiran N (2014) Green synthesized silver and gold nanoparticles for colorimetric detection of Hg2+, Pb2+, and Mn2+ in aqueous medium. ACS Sustain Chem Eng 2(4):887–896
Lei YM, Huang WX, Zhao M, Chai YQ, Yuan R, Zhuo Y (2015) Electrochemiluminescence resonance energy transfer system: mechanism and application in ratiometric aptasensor for lead ion. Anal Chem 87(15):7787–7794
Kashi MB, Silva SM, Yang Y, Goncales VR, Parker SG, Barfidokht A, Ciampi S, Gooding JJ (2017) Light-activated electrochemistry without surface-bound redox species. Electrochim Acta 251:250–255
Sun QW, Wang JK, Tang MH, Huang LM, Zhang ZY, Liu C, Lu XH, Hunter KW, Chen GS (2017) A new electrochemical system based on a flow-field shaped solid electrode and 3D-printed thin-layer flow cell: detection of Pb2+ ions by continuous flow accumulation square-wave anodic stripping voltammetry. Anal Chem 89(9):5024–5029
Hu Q, Kong JM, Han DX, Niu L, Zhang XL (2019) Electrochemical DNA Biosensing via Electrochemically Controlled Reversible Addition–Fragmentation Chain Transfer Polymerization. ACS Sens 4(1):235–241
Ma L, Guo T, Pan SL, Zhang YH (2018) A fluorometric aptasensor for patulin based on the use of magnetized graphene oxide and DNase I-assisted target recycling amplification. Microchim Acta 185(10):487–492
Han C, Li RG, Li H, Liu S, Xu CG, Wang JF, Wang Y, Huang JD (2017) Ultrasensitive voltammetric determination of kanamycin using a target-triggered cascade enzymatic recycling couple along with DNAzyme amplification. Microchim Acta 184(8):2941–2948
Zhang JP, Chao LC, Zhi X, Ramon GR, Liu YL, Zhang CL, Pan F, Cui DX (2016) Hairpin DNA-templated silver nanoclusters as novel beacons in strand displacement amplification for microRNA detection. Anal Chem 88(2):1294–1302
Li MX, Xu CH, Zhang N, Qian GS, Zhao WW, Xu JJ, Chen HY (2018) Exploration of the kinetics of toehold-mediated strand displacement via plasmon rulers. ACS Nano 12(7):3341–3350
Chen F, Zhao Y, Fan CH, Zhao YX (2015) Mismatch extension of DNA polymerases and high-accuracy single nucleotide polymorphism diagnostics by gold nanoparticle-improved isothermal amplification. Anal Chem 87(17):8718–8723
Yao Q, Wang YQ, Wang J, Chen SM, Liu HY, Jiang ZR, Zhang XE, Liu SM, Yuan Q, Zhou X (2018) An ultrasensitive diagnostic biochip based on biomimetic periodic nanostructure-assisted rolling circle amplification. ACS Nano 12(7):6777–6783
Zhao WA, Ali MM, Brook MA, Li YF (2008) Rolling circle amplification: applications in nanotechnology and biodetection with functional nucleic acids. Angew Chem Int Ed 47:6330–6337
Chen Y, Xu J, Su J, Xiang Y, Yuan R, Chai YQ (2012) In situ hybridization chain reaction amplification for universal and highly sensitive electrochemiluminescent detection of DNA. Anal Chem 84(18):7750–7755
He Z, Cai Y, Yang Z, Li P, Lei H, Liu W, Liu Y (2019) A dual-signal readout enzyme-free immunosensor based on hybridization chain reaction-assisted formation of copper nanoparticles for the detection of microcystin-LR. Biosens Bioelectron 126:151–159
Cao HM, Zhou X, Zeng Y (2019) Microfluidic exponential rolling circle amplification for sensitive microRNA detection directly from biological samples. Sensor Actuat B-Chem 279:447–457
Reid MS, Rebecca E, Paliwoda RE, Zhang HQ, Le XC (2018) Reduction of background generated from template-template hybridizations in the exponential amplification reaction. Anal Chem 90(18):11033–11039
Zhang M, Wang Y, Yuan S, Sun X, Huo B, Bai J, Gao Z (2019) Competitive fluorometric assay for the food toxin T-2 by using DNA-modified silver nanoclusters, aptamer-modified magnetic beads, and exponential isothermal amplification. Microchim Acta 186(4):219–225
Yu CY, Yin BC, Wang SL, Xu ZG, Ye BC (2014) Improved ligation-mediated PCR method coupled with T7 RNA polymerase for sensitive DNA detection. Anal Chem 86(15):7214–7218
Du WF, Ge JH, Li JJ, Tang LJ, Yu RQ, Jiang JH (2019) Single-step, high-specificity detection of single nucleotide mutation by primer-activatable loop-mediated isothermal amplification (PA-LAMP). Anal Chim Acta 1050:132–138
Cai S, Jung C, Bhadra S, Ellington A (2018) Phosphorothioated primers lead to loop-mediated isothermal amplification at low temperatures. Anal Chem 90(14):8290–8294
Du YC, Cui YX, Li XY, Sun GY, Zhang YP, Tang AN, Kong DM (2018) Terminal deoxynucleotidyl transferase and T7 exonuclease-aided amplification strategy for ultrasensitive detection of uracil-DNA glycosylase. Anal Chem 90(14):8629–8634
Yang F, Yang X, Wang YZ, Qin Y, Liu X, Yan XQ, Zou K, Ning Y, Zhang GJ (2014) Template-independent, in situ grown DNA nanotail enabling label-free femtomolar chronocoulometric detection of nucleic acids. Anal Chem 86(23):11905–11912
Xie SB, Dong YW, Yuan YL, Chai YQ, Yuan R (2016) Ultrasensitive lipopolysaccharides detection based on doxorubicin conjugated N-(Aminobutyl)-N-(ethylisoluminol) as electrochemiluminescence indicator and self-assembled tetrahedron DNA dendrimers as nanocarriers. Anal Chem 88(10):5218–5224
Niu XF, Zhong YB, Chen R, Wang F, Liu YJ, Luo D (2018) A “turn-on” fluorescence sensor for Pb2+ detection based on graphene quantum dots and gold nanoparticles. Sensor Actuat B-Chem 255:1577–1581
Wang Y, Wang J, Yang F, Yang XR (2010) Spectrophotometric detection of lead(II) ion using unimolecular peroxidase-like deoxyribozyme. Microchim Acta 171:195–201
Chai F, Wang CG, Wang TT, Li L, Su ZM (2010) Colorimetric detection of Pb2+ using glutathione functionalized gold nanoparticles. ACS Appl Mater Interfaces 2:1466–1470
Lidia M, Crina S, Maria C, Florina P, Marcela CR, Stefan G, Stela P (2017) Electrochemical platform based on nitrogendoped graphene/chitosan nanocomposite for selective Pb2+ detection. Nanotechnology 28:114001–1140012
Huang ZJ, Chen JM, Luo ZW, Wang XQ, Duan YX (2019) Label-free and enzyme-free colorimetric detection of Pb2+ based on RNA cleavage and annealing-accelerated hybridization chain reaction. Anal Chem 917:4806–4813
Lu HZ, Xu SF, Liu JQ (2019) One pot generation of blue and red carbon dots in one binary solvent system for dual channel detection of Cr3+ and Pb2+ based on ion imprinted fluorescence polymers. ACS Sens 4:1917–1924
Cui L, Wu J, Li Jie JHX (2015) Electrochemical sensor for lead cation sensitized with a DNA functionalized porphyrinic metal-organic framework. Anal Chem 87:10635–10641
Song XL, Wang Y, Liu S, Zhang X, Wang JF, Wang HW, Zhang FF, Yu JH, Huang JD (2019) A triply amplified electrochemical lead(II) sensor by using a DNAzyme and via formation of a DNA-gold nanoparticle network induced by a catalytic hairpin assembly. Microchim Acta 186:559–566
Shahdordizadeh M, Yazdian-Robati R, Ansari N, Ramezani M, Abnous K, Taghdisi SM (2018) An aptamer-based colorimetric lead(II) assay based on the use of gold nanoparticles modified with dsDNA and exonuclease I. Microchim Acta 185:151–156
Ravikumar A, Panneerselvam P, Radhakrishnan K (2018) Fluorometric determination of lead(II) and mercury(II) based on their interaction with a complex formed between graphene oxide and a DNAzyme. Microchim Acta 185:2–9
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (XDJK2019B022, SWU117045), the Chongqing Research Program of Basic Research and Frontier Technology (cstc2018jcyjA0797) and the national key research and development plan of China (2018YFD0800600).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, L., Deng, H., Yuan, R. et al. Electrochemical lead(II) biosensor by using an ion-dependent split DNAzyme and a template-free DNA extension reaction for signal amplification. Microchim Acta 186, 709 (2019). https://doi.org/10.1007/s00604-019-3857-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3857-z