Abstract
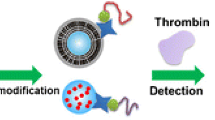
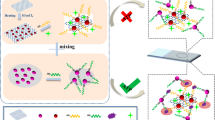
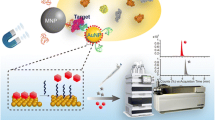
It is known that the intensity of surface-enhanced Raman scattering (SERS) of monomeric gold nanoparticles (GNPs) is insufficient for ultrasensitive analysis. The authors describe dimeric GNPs for use in a competitive SERS and aptamer based assay for thrombin. The reagent 1,2-bis(4-pyridyl) ethylene serves as both the coupling agent and the Raman reporter on the GNP dimers. In the presence of thrombin, the hybridization of two aptamers, one attached to the GNP dimers, the other to magnetic nanoparticles, is competitively prevented. This method takes advantage of the unique “hot spots” of the GNP dimers to amplify the Raman signal. This results in an ultra-sensitive thrombin assay when compared to assays using GNP monomers. The limit of detection is as low as 1 fM of thrombin. The Raman intensity, best measured at 1612 cm−1, increases linearly in the 1 fM to 10 nM thrombin concentration range. The method was applied to the determinaiton of thrombin in spiked simulated body fluid and human serum.

This method takes advantage of the unique “hot spots” of the gold nanoparticle dimers to amplify the Raman signal. The dimers are linked to the magnetic nanoparticles via an aptamer. The use of both competitive displacement and magnetic separation greatly improves the sensitivity of the thrombin assay.





Similar content being viewed by others
References
Coughlin SR (2000) Thrombin signalling and protease-activated receptors. Nat 407(6801):258–264
Tasset DM, Kubik MF, Steiner W (1997) Oligonucleotide inhibitors of human thrombin that bind distinct epitopes. J Mol Biol 272(5):688–698
Arai T, Miklossy J, Klegeris A, Guo J, McGeer PL (2006) Thrombin and prothrombin are expressed by neurons and glial cells and accumulate in neurofibrillary tangles in Alzheimer disease brain. J Neuropathol Exp Neurol 65(1):19–25
Becker RC, Spencer FA (1998) Thrombin: structure, biochemistry, measurement, and status in clinical medicine. J Thromb Thrombolysis 5(3):215–229
Bichler J, Heit JA, Owen WG (1996) Detection of thrombin in huamn blood by ex-vivo hirudin. Thromb Res 84(4):289–294
Chen Z, Tan Y, Zhang C, Yin L, Ma L, Ye N, Qiang H, Lin Y (2014) A colorimetric aptamer biosensor based on cationic polymer and gold nanoparticles for the ultrasensitive detection of thrombin. Biosens Bioelectron 56:46–50
Deng L, Du Y, Xu JJ, Chen HY (2014) An off-on-off electrochemiluminescence approach for ultrasensitive detection of thrombin. Biosens Bioelectron 59:58–63
Li J, Zhong X, Zhang H, Le XC, Zhu JJ (2012) Binding-induced fluorescence turn-on assay using aptamer-functionalized silver nanocluster DNA probes. Anal Chem 84(12):5170–5174
Wang J, Li B, Lu Q, Li X, Weng C, Yan X, Hong J, Zhou X (2019) A versatile fluorometric aptasensing scheme based on the use of a hybrid material composed of polypyrrole nanoparticles and DNA-silver nanoclusters: application to the determination of adenosine, thrombin, or interferon-gamma. Microchim Acta 186(6):356
Wen C, Bi J, Wu L, Zeng J (2018) Aptamer-functionalized magnetic and fluorescent nanospheres for one-step sensitive detection of thrombin. Microchim Acta 185(1):77
Wustholz KL, Henry AI, McMahon JM, Freeman RG, Valley N, Piotti ME, Natan MJ, Schatz GC, Van Duyne RP (2010) Structure-activity relationships in gold nanoparticle dimers and trimers for surface-enhanced Raman spectroscopy. J Am Chem Soc 132:10903–10910
Simonsen JB, Reeler NE, Fossum A, Lerstrup KA, Laursen BW, Nørgaard K (2016) Quantifying and sorting of gold nanoparticle dimers from complex reaction mixtures using flow cytometry. Nano Res 9:3093–3098
Kneipp K, Kneipp H, Itzkan I, Dasari RR, Feld MS (1999) Ultrasensitive chemical analysis by Raman spectroscopy. Chem Rev 99(10):2957–2976
Doering WE, Nie SM (2003) Spectroscopic tags using dyeembedded nanoparticles and surface-enhanced Raman scattering. Anal Chem 75:6171–6176
Ahmadi A, Shirazi H, Pourbagher N, Akbarzadeh A, Omidfar K (2014) An electrochemical immunosensor for digoxin using core-shell gold coated magnetic nanoparticles as labels. Mol Biol Rep 41(3):1659–1668
Jo M, Ahn JY, Lee J, Lee S, Hong SW, Yoo JW, Kang J, Dua P, Lee DK, Hong S, Kim S (2011) Development of single-stranded DNA aptamers for specific bisphenol a detection. Oligonucleotides 21(2):85–91
Wang W, Chen C, Qian M, Zhao XS (2008) Aptamer biosensor for protein detection using gold nanoparticles. Anal Biochem 373(2):213–219
Ozalp VG, Bayramoglu G, Erdem Z, Arica MY (2015) Pathogen detection in complex samples by quartz crystal microbalance sensor coupled to aptamer functionalized core-shell type magnetic separation. Anal Chim Acta 853:533–540
Sun YH, Kong RM, Lu DQ, Zhang XB, Meng HM, Tan W, Shen GL, Yu RQ (2011) A nanoscale DNA-au dendrimer as a signal amplifier for the universal design of functional DNA-based SERS biosensors. Chem Commun 47(13):3840–3842
Zhou Z, Du Y, Dong S (2011) Double-strand DNA-templated formation of copper nanoparticles as fluorescent probe for label-free aptamer sensor. Anal Chem 83(13):5122–5127
Jiang N, Hu Y, Wei W, Zhu T, Yang K, Zhu G, Yu M (2019) Detection of microRNA using a polydopamine mediated bimetallic SERS substrate and a re-circulated enzymatic amplification system. Microchim Acta 186:65
Sun L, Yu C, Irudayaraj J (2007) Surface-enhanced Raman scattering based nonfluorescent probe for multiplex DNA detection. Anal Chem 79(11):3981–3988
Zheng X, Hu P, Cui Y, Zong C, Feng J, Wang X, Ren B (2014) BSA-coated nanoparticles for improved SERS-based intracellular pH sensing. Anal Chem 86(24):12250–12257
Zyubinaa T, Razumova V, Brichkina S, Anisimovb V, Linc S, Mebeld A (2006) Quantum-chemical study of crystal formation of supramolecular silver compounds with trans-1,2-Bis(4-pyridyl)ethylene and their electronic absorption spectra. Russ J Inorg Chem 51(6):925–940
Dufek EJ, Ehlert B, Granger MC, Sandrock TM, Legge SL, Herrmann MG, Meikle AW, Porter MD (2010) Competitive surface-enhanced Raman scattering assay for the 1,25-dihydroxy metabolite of vitamin D3. Analyst 135(11):2811–2817
Bai Y, Feng F, Zhao L, Wang C, Wang H, Tian M, Qin J, Duan Y, He X (2013) Aptamer/thrombin/aptamer-AuNPs sandwich enhanced surface plasmon resonance sensor for the detection of subnanomolar thrombin. Biosens Bioelectron 47:265–270
Chang H, Tang L, Wang Y, Jiang J, Li J (2010) Graphene fluorescence resonance energy transfer Aptasensor for the thrombin detection. Anal Chem 82(6):2341–2346
Li T, Wang E, Dong S (2008) G-quadruplex-based DNAzyme for facile colorimetric detection of thrombin. Chem Commun (31):3654–3656
Wang Q, Zhou Z, Zhai Y, Zhang L, Hong W, Zhang Z, Dong S (2015) Label-free aptamer biosensor for thrombin detection based on functionalized graphene nanocomposites. Talanta 141:247–252
Yoon J, Choi N, Ko J, Kim K, Lee S, Choo J (2013) Highly sensitive detection of thrombin using SERS-based magnetic aptasensors. Biosens Bioelectron 47:62–67
Acknowledgements
This research has been supported by National Natural Science Foundation of China (NSFC) (Grant nos. 21273083 and U1732146) and the Project under Scientific and Technological Planning Grant nos. 2014A040401075 by Guangdong Province and 201805010002 by Guangzhou City.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 467 kb)
Rights and permissions
About this article
Cite this article
Jiang, N., Zhu, T. & Hu, Y. Competitive aptasensor with gold nanoparticle dimers and magnetite nanoparticles for SERS-based determination of thrombin. Microchim Acta 186, 747 (2019). https://doi.org/10.1007/s00604-019-3787-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3787-9