Abstract
The authors describe a method that can significantly improve the performance of impedimetric detection of bacteria. A multifunctional microfluidic chip was designed consisting of interdigitated microelectrodes and a micro-mixing zone with a Tesla structure. This maximizes the coating of bacterial surfaces with nanoparticles and results in improved impedimetric detection. The method was applied to the detection of Escherichia coli O157:H7 (E. coli). Silver enhancement was accomplished by coating E.coli with the cationic polymer diallyldimethylammonium chloride (PDDA) to form positively charged E. coli/PDDA complexes. Then, gold nanoparticles (AuNPs) were added, and the resulting E. coli/PDDA/AuNPs complexes were collected at interdigitated electrodes via positive dielectrophoresis (pDEP). A silver adduct was then formed on the E. coli/PDDA/AuNP complexes by using silver enhancement solutions and by using the AuNPs as catalysts. The combination of pDEP based capture and of using silver adducts reduces impedance by increasing the conductivity of the solution and the double layer capacitance around the microelectrodes. Impedance decreases linearly in the 2 × 103–2 × 105 cfu·mL−1 E. coli concentration range, with a 500 cfu·mL−1 detection limit. Egg shell wash samples and tap water spiked with E. coli were successfully used for validation, and this demonstrates the practical application of this method.

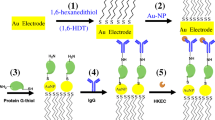
Schematic representation of the AuNP@Ag enhancement method integrated with multifunctional microfluidic chip platform for impedimetric quantitation of bacteria. The method significantly improves the performance of impedimetric detection of bacteria.





Similar content being viewed by others
References
Fabai WCD (2016) Nanofabricated structures and microfluidic devices for bacteria: from techniques to biology. Chem Soc Rev 45:268–280. https://doi.org/10.1039/C5CS00514K
Song Y, Zhang H, Chon CH, Chen S, Pan X, Li D (2010) Counting bacteria on a microfluidic chip. Anal Chim Acta 681(1–2):82–86. https://doi.org/10.1016/j.aca.2010.09.035
Fronczek CF, You DJ, Yoon JY (2013) Single-pipetting microfluidic assay device for rapid detection of Salmonella from poultry package. Biosens Bioelectron 40(1):342–349. https://doi.org/10.1016/j.bios.2012.07.076
Varshney M, Li Y, Srinivasan B, Tung S (2007) A label-free, microfluidics and interdigitated array microelectrode-based impedance biosensor in combination with nanoparticles immunoseparation for detection of Escherichia coli O157:H7 in food samples. Sensors Actuators B Chem 128(1):99–107. https://doi.org/10.1016/j.snb.2007.03.045
Varshney M, Li Y (2007) Interdigitated array microelectrode based impedance biosensor coupled with magnetic nanoparticle-antibody conjugates for detection of Escherichia coli O157:H7 in food samples. Biosens Bioelectron 22(11):2408–2414. https://doi.org/10.1016/j.bios.2006.08.030
Donato SS, Chu V, Prazeres DM, Conde JP (2013) Metabolic viability of Escherichia coli trapped by dielectrophoresis in microfluidics. Electrophoresis 34(4):575–582. https://doi.org/10.1002/elps.201200292
Yang L (2012) A Review of Multifunctions of Dielectrophoresis in Biosensors and Biochips for Bacteria Detection. Anal Lett 45(2–3):187–201. https://doi.org/10.1080/00032719.2011.633182
Yang L, Banada PP, Chatni MR, Seop Lim K, Bhunia AK, Ladisch M, Bashir R (2006) A multifunctional micro-fluidic system for dielectrophoretic concentration coupled with immuno-capture of low numbers of Listeria monocytogenes. Lab Chip 6(7):896–905. https://doi.org/10.1039/b607061m
Yang L (2009) Dielectrophoresis assisted immuno-capture and detection of foodborne pathogenic bacteria in biochips. Talanta 80(2):551–558. https://doi.org/10.1016/j.talanta.2009.07.024
He X, Hu C, Guo Q, Wang K, Li Y, Shangguan J (2013) Rapid and ultrasensitive Salmonella Typhimurium quantification using positive dielectrophoresis driven on-line enrichment and fluorescent nanoparticleslabel. Biosens Bioelectron 42:460–466. https://doi.org/10.1016/j.bios.2012.11.020
Suehiro J, Shutou M, Hatano T, Hara M (2003) High sensitive detection of biological cells using dielectrophoretic impedance measurement method combined with electropermeabilization. Sensors Actuators B Chem 96(1–2):144–151. https://doi.org/10.1016/s0925-4005(03)00517-3
Yang L (2008) Electrical impedance spectroscopy for detection of bacterial cells in suspensions using interdigitated microelectrodes. Talanta 74(5):1621–1629. https://doi.org/10.1016/j.talanta.2007.10.018
Suehiro J, Ohtsubo A, Hatano T, Hara M (2006) Selective detection of bacteria by a dielectrophoretic impedance measurement method using an antibody-immobilized electrode chip. Sensors Actuators B Chem 119(1):319–326. https://doi.org/10.1016/j.snb.2005.12.027
Kim S, Yu G, Kim T, Shin K, Yoon J (2012) Rapid bacterial detection with an interdigitated array electrode by electrochemical impedance spectroscopy. Electrochim Acta 82:126–131. https://doi.org/10.1016/j.electacta.2012.05.131
Wang R, Ni Y, Xu Y, Jiang Y, Dong C, Chuan N (2015) Immuno-capture and in situ detection of Salmonella typhimurium on a novel microfluidic chip. Anal Chim Acta 853:710–717. https://doi.org/10.1016/j.aca.2014.10.042
Chen ZP, Peng ZF, Luo Y, Qu B, Jiang JH, Zhang XB, Shen GL, Yu RQ (2007) Successively amplified electrochemical immunoassay based on biocatalytic deposition of silver nanoparticles and silver enhancement. Biosens Bioelectron 23(4):485–491. https://doi.org/10.1016/j.bios.2007.06.005
Chen X, Zhang L (2017) A review on micromixers actuated with magnetic nanomaterials. Microchim Acta 184(10):3639–3649. https://doi.org/10.1007/s00604-017-2462-2
K-L S, Huang H-H, Chang TC, Lin H-P, Lin Y-C, Chen W-T (2008) An immunoassay using an electro-microchip, nanogold probe and silver enhancement. Microfluid Nanofluid 6(1):93–98. https://doi.org/10.1007/s10404-008-0299-z
Yeh CH, Chang YH, Chang TC, Lin HP, Lin YC (2010) Electro-microchip DNA-biosensor for bacteria detection. Analyst 135(10):2717–2722. https://doi.org/10.1039/c0an00186d
Yeh C-H, Chen W-T, Lin H-P, Chang T-C, Lin Y-C (2009) Development of an immunoassay based on impedance measurements utilizing an antibody–nanosilver probe, silver enhancement, and electro-microchip. Sensors Actuators B Chem 139(2):387–393. https://doi.org/10.1016/j.snb.2009.03.029
Yeh CH, Huang HH, Chang TC, Lin HP, Lin YC (2009) Using an electro-microchip, a nanogold probe, and silver enhancement in an immunoassay. Biosens Bioelectron 24(6):1661–1666. https://doi.org/10.1016/j.bios.2008.08.039
Lin L, Liu Y, Tang L, Li J (2011) Electrochemical DNA sensor by the assembly of graphene and DNA-conjugated gold nanoparticles with silver enhancement strategy. Analyst 136(22):4732–4737. https://doi.org/10.1039/c1an15610a
Sabhachandani P, Sarkar S, Zucchi PC, Whitfield BA, Kirby JE, Hirsch EB, Konry T (2017) Integrated microfluidic platform for rapid antimicrobial susceptibility testing and bacterial growth analysis using bead-based biosensor via fluorescence imaging. Microchim Acta 184(12):4619–4628. https://doi.org/10.1007/s00604-017-2492-9
Jang H, Lee P, Kim S, Kim SM, Jeon T-J (2017) An antibacterial microfluidic system with fish gill structure for the detection of Staphylococcus via enzymatic reaction on a chromatic polydiacetylene material caused by lysostaphin. Microchim Acta 184(11):4563–4569. https://doi.org/10.1007/s00604-017-2517-4
Hong CC, Choi JW, Ahn CH (2004) A novel in-plane passive microfluidic mixer with modified Tesla structures. Lab Chip 4(2):109–113. https://doi.org/10.1039/b305892a
Ravindranath SP, Wang Y, Irudayaraj J (2011) SERS driven cross-platform based multiplex pathogen detection. Sensors Actuators B Chem 152:183–190. https://doi.org/10.1016/j.snb.2010.12.005
Cho Il-Hoon, Radadia AD, Farrokhzad K, Ximenes E, Euiwon B, Singh AK, Oliver H, Ladisch M, Bhunia A, Applegate B, Mauer L, Bashir R, Irudayaraj J (2014) Nano/Micro and Spectroscopic Approaches to Food Pathogen Detection. Annu Rev Anal Chem 7:65–88. https://doi.org/10.1146/annurev-anchem-071213-020249
Dickson JS, Koohmarare M (1989) Cell Surface Charge Characteristics and Their Relationship to Bacterial Attachment to Meat Surfaces. Appl Environ Microbiol 55(4):5
Wilson WW, Wade MM, Holman SC, Champlin FR (2001) Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J Microbiol Methods 43(3):153–164. https://doi.org/10.1016/s0167-7012(00)00224-4
Lackie PM (1996) Immunogold silver staining for light microscopy. Histochem Cell Biol 106:9–17
Acknowledgements
We thank Dr. Zhongwu Zhou and PabloVega for their critical reading of the manuscript. The work was financially supported by National Natural Science Foundation of China (No 21375156); National High Technology Research and Development Program of China (Ministry of Science and Technology 863 Plan) (No 2015AA021104); Frontier Research Key Projects of Chongqing Science and Technology Committee, [cstc2015jcyjBX0010]; Scientific and Technical Innovation Projects for People’s Livelihood of Chongqing Science and Technology Committee, [cstc2015shms,zx00014]; Benefit Projects for People’s Livelihood by Science and Technology, Chongqing Science and Technology Committee 【cstc2015jcsf8001】,2015.07-2017.07; Fundamental Research Funds for the Central Universities (Fund for Brain Science), (No.10611CDJXZ238826) Partial support from the Center for Food Safety Engineering, Agricultural Research Service, under Agreement No. 1935-42000-049-00D at Purdue University is appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 817 kb)
Rights and permissions
About this article
Cite this article
Wang, R., Xu, Y., Sors, T. et al. Impedimetric detection of bacteria by using a microfluidic chip and silver nanoparticle based signal enhancement. Microchim Acta 185, 184 (2018). https://doi.org/10.1007/s00604-017-2645-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2645-x