Abstract
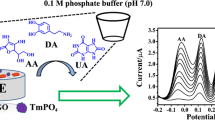
The authors describe an amperometric sensor for dopamine (DA) which has a working potential as low as +0.02 V (vs. SCE). It makes use of a nanocomposite consisting of reduced graphene oxide (rGO) and manganic manganous oxide (Mn3O4) in a film of Nafion on gold nanoparticles deposited on a gold electrode. The composite was characterized by X-ray powder diffractometry (XRD), Fourier transform infrared spectroscopy (FTIR) and field emission scanning electron microscopy (FE-SEM). The electrochemical properties of the modified electrode were investigated by cyclic voltammetry, electrochemical impedance spectroscopy (EIS) and amperometric methods. After method optimization, the amperogram displays a linear range extending from 1.0 μmol·L−1 to 1.45 mmol·L−1 with a limit of detection as low as 0.25 μmol·L−1 (at an S/N ratio of 3). The modified electrode was employed for the determination of DA in injection solution samples with satisfactory results.

Schematic of an electrochemical sensor based on a gold electrode (GE) sensitized with a nanocomposite consisting of reduced graphene oxide (rGO) and manganic manganous oxide (Mn3O4) in a film of Nafion supported gold nanoparticles (Au).





Similar content being viewed by others
References
Xu YQ, Hun X, Liu F, Wen XL, Luo XL (2015) Aptamer biosensor for dopamine based on a gold electrode modified with carbon nanoparticles and thionine labeled gold nanoparticles as probe. Microchim Acta 182:1797–1802. doi:10.1007/s00604-015-1509-5
Zhang QL, Feng JX, Wang AJ, Wei J, Lv ZY, Feng JJ (2015) A glassy carbon electrode modified with porous gold nanosheets for simultaneous determination of dopamine and acetaminophen. Microchim Acta 182:589–595. doi:10.1007/s00604-014-1363-x
Hsieh YS, Hong BD, Lee CL (2016) Non-enzymatic sensing of dopamine using a glassy carbon electrode modified with a nanocomposite consisting of palladium nanocubes supported on reduced graphene oxide in a nafion matrix. Microchim Acta 183:905–910. doi:10.1007/s00604-015-1668-4
Yan XY, Gu Y, Li C, Tang L, Zheng B, Li YR, Zhang ZQ, Yang M (2016) Synergetic catalysis based on the proline tailed metalloporphyrin with graphene sheet as efficient mimetic enzyme for ultrasensitive electrochemical detection of dopamine. Biosens Bioelectron 77:1032–1038. doi:10.1016/j.bios.2015.10.085
Sanghavi BJ, Wolfbeis OS, Hirsch T, Swami NS (2015) Nanomaterial-based electrochemical sensing of neurological drugs and neurotransmitters. Microchim Acta 182:1–41. doi:10.1007/s00604-014-1308-4
Wu BN, Miao CC, Yu LL, Wang ZY, Huang CS, Jia NQ (2014) Sensitive electrochemiluminescence sensor based on ordered mesoporous carbon composite film for dopamine. Sensors Actuators B Chem 195:22–27. doi:10.1016/j.snb.2014.01.012
Carrera V, Sabater E, Vilanova E, Sogorb MA (2007) A simple and rapid HPLC-MS method for the simultaneous determination of epinephrine, norepinephrine, dopamine and 5-hydroxytryptamine: application to the secretion of bovine chromaffin cell cultures. J Chromatogr B 847:88–94. doi:10.1016/j.jchromb.2006.09.032
Naccarato A, Gionfriddo E, Sindona G, Tagarelli A (2014) Development of a simple and rapid solid phase microextraction-gas chromatography-triple quadrupole mass spectrometry method for the analysis of dopamine, serotonin and norepinephrine in human urine. Anal Chim Acta 810:17–24. doi:10.1016/j.aca.2013.11.058
Wang HB, Zhang HD, Chen Y, Huang KJ, Liu YM (2015) A label-free and ultrasensitive fluorescent sensor for dopamine detection based on double-stranded DNA templated copper nanoparticles. Sensors Actuators B Chem 220:146–153. doi:10.1016/j.snb.2015.05.055
Lin FE, Gui C, Wen W, Bao T, Zhang XH, Wang SF (2016) Dopamine assay based on an aggregation-induced reversed inner filter effect of gold nanoparticles on the fluorescence of graphene quantum dots. Talanta 158:292–298. doi:10.1016/j.talanta.2016.05.062
Hou SR, Zheng N, Feng HY, Li XJ, Yuan ZB (2008) Determination of dopamine in the presence of ascorbic acid using poly (3,5-dihydroxy benzoic acid) film modified electrode. Anal Biochem 381:179–184. doi:10.1016/j.ab.2008.03.055
Fernandes SC, Vieira IC, Peralta RA, Neves A (2010) Development of a biomimetic chitosan film-coated gold electrode for determination of dopamine in the presence of ascorbic acid and uric acid. Electrochim Acta 55:7152–7157. doi:10.1016/j.electacta.2010.06.062
Huang Y, Cheng CM, Tian XQ, Zheng BZ, Li Y, Yuan HY, Xiao D, Choi MMF (2013) Low-potential amperometric detection of dopamine based on MnO2 nanowires/chitosan modified gold electrode. Electrochim Acta 89:832–839. doi:10.1016/j.electacta.2012.11. 071
Sajid M, Nazal MK, Mansha M, Alsharaa A, Jillani SMJ, Basheer C (2016) Chemically modified electrodes for electrochemical detection of dopamine in the presence of uric acid and ascorbic acid: a review. TrAC Trends Anal Chem 76:15–29. doi:10.1016/j.trac.2015.09.006
Li Y, Lin HC, Peng H, Qi RJ, Luo CH (2016) A glassy carbon electrode modified with MoS2 nanosheets and poly(3,4-ethylenedioxythiophene) for simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. Microchim Acta 183:2517–2523. doi:10.1007/s00604-016-1897-1
Jin JY, Mei H, Wu HM, Wang SF, Xia QH, Ding Y (2016) Selective detection of dopamine based on Cu2O@Pt core-shell nanoparticles modified electrode in the presence of ascorbic acid and uric acid. J Alloys Compd 689:174–181. doi:10.1016/j.jallcom.2016.07.322
Numan A, Shahid MM, Omar FS, Ramesh K, Ramesh S (2017) Facile fabrication of cobalt oxide nanograin-decorated reduced graphene oxide composite as ultrasensitive platform for dopamine detection. Sensors Actuators B Chem 238:1043–1051. doi:10.1016/j.snb.2016.07.111
Ejaz A, Joo Y, Jeon SW (2017) Fabrication of 1,4-bis(aminomethyl)benzene and cobalt hydroxide @graphene oxide for selective detection of dopamine in the presence of ascorbic acid and serotonin. Sensors Actuators B Chem 240:297–307. doi:10.1016/j.snb.2016.08.171
Sivasubramanian R, Biji P (2016) Preparation of copper (I) oxide nanohexagon decorated reduced graphene oxide nanocomposite and its application in electrochemical sensing of dopamine. Mater Sci Eng B 210:10–18. doi:10.1016/j.mseb.2016.04.018
Khudaish EA, Al-Nofli F, Rather JA, Al-Hinaai M, Laxman K, Kyaw HH, Al-Harthy S (2016) Sensitive and selective dopamine sensor based on novel conjugated polymer decorated with gold nanoparticles. J Electroanal Chem 761:80–88. doi:10.1016/j.jelechem.2015.12.011
Jia LP, Zhou YX, Jiang YM, Zhang AH, Li X, Wang CM (2016) A novel dopamine sensor based on Mo doped reduced graphene oxide/polyimide composite membrane. J Alloys Compd 685:167–174. doi:10.1016/j.jallcom.2016.05.239
Zhang DD, Li LZ, Ma WN, Chen X, Zhang YM (2017) Electrodeposited reduced graphene oxide incorporating polymerization of L-lysine on electrode surface and its application in simultaneous electrochemical determination of ascorbic acid, dopamine and uric acid. Mater Sci Eng C 70:241–249. doi:10.1016/j.msec.2016.08.078
Vilian ATE, An SY, Choe SR, Kwak CH, Huh YS, Lee JH, Han YK (2016) Fabrication of 3D honeycomb-like porous polyurethane-functionalized reduced graphene oxide for detection of dopamine. Biosens Bioelectron 86:122–128. doi:10.1016/j.bios.2016.06.022
Li XJ, Song ZW, Zhao Y, Wang Y, Zhao XC, Liang MH, Chu WG, Jiang P, Liu Y (2016) Vertically porous nickel thin film supported Mn3O4 for enhanced energy storage performance. J Colloid Interface Sci 483:17–25. doi:10.1016/j.jcis.2016.08.006
Xu JS, Fan XM, Xia Q, Shao ZM, Pei B, Yang ZH, Chen ZX, Zhang WX (2016) A highly atom-efficient strategy to synthesize reduced graphene oxide-Mn3O4 nanoparticles composites for supercapacitors. J Alloys Compd 685:949–956. doi:10.1016/j.jallcom.2016.06.247
Zhen MM, Zhang Z, Ren QT, Liu L (2016) Room-temperature synthesis of ultrathin Mn3O4 nanosheets as anode materials for lithium-ion batteries. Mater Lett 177:21–24. doi:10.1016/j.matlet.2016.04.156
Singh N, Ali MA, Suresh K, Agrawal VV, Rai P, Sharma A, Malhotra BD, John R (2016) In-situ electrosynthesized nanostructured Mn3O4-polyaniline nanofibers-biointerface for endocrine disrupting chemical detection. Sensors Actuators B Chem 236:781–793. doi:10.1016/j.snb.2016.06.050
Yusoff N, Pandikumar A, Ramaraj R, Lim HN, Huang NM (2015) Gold nanoparticle based optical and electrochemical sensing of dopamine. Microchim Acta 182:2091–2114. doi:10.1007/s00604-015-1609-2
Khudaish EA, Rather JA (2016) Electrochemical studies of dopamine under stagnant and convective conditions at a sensor based on gold nanoparticles hosted in poly(triaminopyrimidine). J Electroanal Chem 776:206–212. doi:10.1016/j.jelechem.2016.06.041
Li SJ, Deng DH, Shi Q, Liu SR (2012) Electrochemical synthesis of a graphene sheet and gold nanoparticle-based nanocomposite, and its application to amperometric sensing of dopamine. Microchim Acta 177:325–331. doi:10.1007/s00604-012-0782-9
Salimi A, Abdi K, Khayatian GR (2004) Amperometric detection of dopamine in the presence of ascorbic acid using a nafion coated glassy carbon electrode modified with catechin hydrate as a natural antioxidant. Microchim Acta 144:161–169. doi:10.1007/s00604-003-0048-7
Yang X, Xiao FB, Lin HW, Wu F, Chen DZ, Wu ZY (2013) A novel H2O2 biosensor based on Fe3O4-au magnetic nanoparticles coated horseradish peroxidase and graphene sheets-nafion film modified screen-printed carbon electrode. Electrochim Acta 109:750–755. doi:10.1016/j.electacta.2013.08.011
Yang X, Ouyang YJ, Wu F, Hu YJ, Ji Y, Wu ZY (2017) Size controllable preparation of gold nanoparticles loading on graphene sheets@cerium oxide nanocomposites modified gold electrode for nonenzymatic hydrogen peroxide detection. Sensors Actuators B Chem 238:40–47. doi:10.1016/j.snb.2016.07.016
Cao X, Cai XL, Wang N (2011) Selective sensing of dopamine at MnOOH nanobelt modified electrode. Sensors Actuators B Chem 160:771–776. doi:10.1016/j.snb.2011.08.061
Abdelwahab AA, Lee HM, Shim YB (2009) Selective determination of dopamine with a cibacron blue/poly-1,5-diaminonaphthalene composite film. Anal Chim Acta 650:247–253. doi:10.1016/j.aca.2009.07.054
Adekunle AS, Agboola BO, Pillay J, Ozoemena KI (2010) Electrocatalytic detection of dopamine at single-walled carbon nanotubes-iron (III) oxide nanoparticles platform. Sensors Actuators B Chem 148:93–102. doi:10.1016/j.snb.2010.03.088
Kumar MK, Prataap RV, Mohan S, Jha SK (2016) Preparation of electro-reduced graphene oxide supported walnut shape nickel nanostructures, and their application to selective detection of dopamine. Microchim Acta 183:1759–1768. doi:10.1007/s00604-016-1806-7
Rao D, Zhang X, Sheng Q, Zheng J (2016) Highly improved sensing of dopamine by using glassy carbon electrode modified with MnO2, graphene oxide, carbon nanotubes and gold nanoparticles. Microchim Acta 183:2597–2604. doi:10.1007/s00604-016-1902-8
Acknowledgements
The work was supported by the National Natural Science Foundation of China (No. 51672104 and No. 21675045), the Natural Science Foundation of Hunan Province (No. 2016JJ4071, No. 2017JJ2198) and the Foundation of Hunan Provincial Education Department (No.16B205 and No. 17C1148).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 1423 kb)
Rights and permissions
About this article
Cite this article
Yao, Z., Yang, X., Niu, Y. et al. Voltammetric dopamine sensor based on a gold electrode modified with reduced graphene oxide and Mn3O4 on gold nanoparticles. Microchim Acta 184, 2081–2088 (2017). https://doi.org/10.1007/s00604-017-2210-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2210-7