Abstract
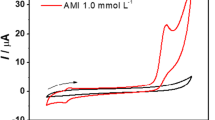
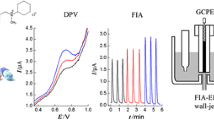
The authors describe a method for amperometric determination of chloramine-T that is based on the indirect detection of chloramine-T by detecting p-quinone imine (p-QI) that is generated by oxidation of p-aminophenylboronic acid by chloramine-T. p-QI can be detected with excellent selectivity and at low potential by using a glassy carbon electrode. Hence, the method displays attractive features such as high sensitivity, wide detection range and excellent selectivity. The electrode has two linear responses in the 50 nM to 100 μM concentration range and a 6 nM detection limit. Compared to other electrochemical methods, this assay has a detection limit that is better by three orders of magnitude. The relative standard deviation is 3.4% for the determination of 10 μM of the medical chloramine-T sample, and the recovery of a samples containing chloramine-T at a level of 10 μM is 115%.

Highly sensitive electrochemical detection of chloramine-T is achieved based on the reaction of chloramine-T with p-aminophenylboronic acid with a detection limit of 6 nM.




Similar content being viewed by others
References
Manjunatha AS, Dakshayani S, Vaz N, Puttaswamy (2015) Oxidative conversion of anilines to azobenzenes with alkaline chloramine-T. Korean J Chem Eng 33(2):697–706
Sen S, Chattopadhyay S, Sarkar P (2015) Electrochemical sensing of tea polyphenols by chloramine-T modified electrodes: a new approach. J Electrochem Soc 163(3):B49–B55
Agnihotri G (2005) Chloramine-T (sodium N-chloro- p – toluenesulfonamide). Synlett (18):2857–2858
Vinod KN, Puttaswamy, Ninge Gowda KN (2009) Mechanistic aspects for the oxidation of sunset yellow dye by chloramine-T in presence of perchloric acid and in sodium hydroxide medium catalyzed by Os(VIII): a spectrophotometric kinetic approach. Inorg Chim Acta 362:2044–2051
Sukhdev AS, Puttaswamy (2012) Kinetic and mechanistic investigation of S-oxidation of ranitidine hydrochloride with chloramine-T in acid and alkaline media. Prog React Kinet Mec 37(1):42–58 (17)
Baranowska I, Bijak K (2010) Differential pulse voltammetry in analysis of disinfectants — 2-mercaptobenzothiazole, 4-chloro-3-methylphenol, triclosan, chloramine-T. Open Chem 8(6):1266–1272
Krishnamurthy N, Pullarao Y, Satyanarayana V (1974) Cacotheline as redox indicator in the volumetric determination of tin(II), titanium(III) and ascorbic acid with chloramine T. Fresenius. Z Anal Chem 272(5):367–367
Catalá Icardo M (2000) Flow injection biamperometric determination of chloramine-T in environmental, pharmaceutical and veterinary samples. Anal Chim Acta 407:187–192
Xi H, Ma Q, Wang C (2011) Determination of chloramine T in cosmetics by ultrasound-assisted Hydrolyzation-LC and validation by LC-MS/MS. J Instrumental Anal 30(8):907–911
Leggett DJ, Chen NH, Mahadevappa DS (1982) Flow injection analysis of aromatic sulphonyl Haloamines. Fresenius Z Anal Chem 311:687–690
Zhao W, Chen R, Dai P, Li X, Xu J, Chen H (2014) A general strategy for photoelectrochemical immunoassay using an enzyme label combined with a CdS quantum dot/TiO(2) nanoparticle composite electrode. Anal Chem 86(23):11513–11516
Park S, Kim J, Ock H, Dutta G, Seo J, Shin EC, Yang H (2015) Sensitive electrochemical detection of vaccinia virus in a solution containing a high concentration of L-ascorbic acid. Analyst 140(16):5481–5487
Selvaraju T, Das J, Han SW, Yang H (2008) Ultrasensitive electrochemical immunosensing using magnetic beads and gold nanocatalysts. Biosens Bioelectron 23(7):932–938
Amene Amani SK, Davood N (2012) Electrochemical oxidation of 4-(piperazin-1-yl)phenols in the presence of indole derivatives: the unique regioselectivity in the synthesis of highly conjugated bisindolyl- p-quinone derivatives. J Electroanal Chem 670:36–41
Liang F, Hu L, Li Y, Majeed S, Li H, Cai H, Yang X, Xu G (2013) Low-potential determination of hydrogen peroxide, uric acid and uricase based on highly selective oxidation of p-hydroxyphenylboronic acid by hydrogen peroxide. Sensor Actuat B-Chem 178:144–148
Hu L, Han S, Liu Z, Parveen S, Yuan Y, Xu G (2011) A versatile strategy for electrochemical detection of hydrogen peroxide as well as related enzymes and substrates based on selective hydrogen peroxide-mediated boronate deprotection. Electrochem Commun 13(12):1536–1538
La M, Feng Y, Yang C, Chen C (2014) Performances of p-aminophenol redox cycling by thiols and Tris(2-carboxyethyl)phosphine on Cysteamine- and 3- Mercaptopropionic acid-covered gold electrodes. Int J Electrochem Sci 9:6985–6992
Jagotamoy D, Kyungmin J, Jae Wook L, Yang H (2007) Electrochemical Immunosensor using p-aminophenol redox cycling by hydrazine combined with a low background current. Anal Chem 79:2790–2796
Xia N, Ma F, Zhao F, He Q, Du J, Li S, Chen J, Liu L (2013) Comparing the performances of electrochemical sensors using p-aminophenol redox cycling by different reductants on gold electrodes modified with self-assembled monolayers. Electrochim Acta 109:348–354
Tang J, Tang D, Su B, Huang J, Qiu B, Chen G (2011) Enzyme-free electrochemical immunoassay with catalytic reduction of p-nitrophenol and recycling of p-aminophenol using gold nanoparticles-coated carbon nanotubes as nanocatalysts. Biosens Bioelectron 26(7):3219–3226
Tang J, Tang D, Niessner R, Knopp D (2011) A novel strategy for ultra-sensitive electrochemical immunoassay of biomarkers by coupling multifunctional iridium oxide (IrO(x)) nanospheres with catalytic recycling of self-produced reactants. Anal Bioanal Chem 400(7):2041–2051
Walter A, Wu J, Flechsig GU, Haake DA, Wang J (2011) Redox cycling amplified electrochemical detection of DNA hybridization: application to pathogen E. coli bacterial RNA. Anal Chem Acta 689(1):29–33
Jia FF, Zhong H, Li XR, Zhu FX, Liu GQ, Cheng ZP, Guo LP (2015) Research on novel nonenzymatic ECL sensor using Au-HS/SO3H-PMO (et) nanocomposites for glucose detection. J Electroanal Chem 758:93–99
Zhu S, Fan L, Liu X, Shi L, Li H, Han S, Xu G (2008) Determination of concentrated hydrogen peroxide at single-walled carbon nanohorn paste electrode. Electrochem Commun 10(5):695–698
Acknowledgements
This project was kindly supported by the National Natural Science Foundation of China (No. 21475123), Jilin Provincial Science and Technology Department (No. 2015020403YY), Changchun city technology bureau (No. 14KG046), the Chinese Academy of Sciences (CAS)-the Academy of Sciences for the Developing World (TWAS) President’s Fellowship Programme, CAS-TWAS Postgraduate Fellowship and CAS President’s International Fellowship Initiative (PIFI).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 73 kb)
Rights and permissions
About this article
Cite this article
Hui, P., Gao, W., Nsabimana, J. et al. Amperometric detection of chloramine-T based on its reaction with p-aminophenylboronic acid. Microchim Acta 184, 687–691 (2017). https://doi.org/10.1007/s00604-016-2060-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-2060-8