Abstract
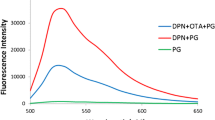
The authors describe an aptamer based assay for determination of ractopamine (RAC) by using PicoGreen (PG) as a fluorescent probe specific for dsDNA. In the absence of RAC, the aptamer forms a duplex structure with a complementary sequence that results in enhanced PG fluorescence. Upon binding to RAC, the aptamer undergoes a structural switch. This reduces the number of DNA duplexes formed and causes a reduction of fluorescence intensity of PG as measured at excitation/emission wavelengths of 480/520 nm. Under optimized conditions, the dynamic calibration plot covers the 50 pM to 50 μM concentration range, with a 50 pM detection limit. This meets the safety supervision regulations of the European Commission in terms of residue limits of RAC in food. The method displays high selectivity over other β-adrenergic agonists including clenbuterol, dopamine and salbutamol. The assay was successfully applied to samples of swine urine at spiking levels of 7.4 nM, 22.2 nM and 37 nM. Average recoveries ranged from 95 to 110%, with an RSD of <1.5%. The method is expected to represent a promising tool for simple, rapid and sensitive on-site detection of RAC in animal products.

An aptamer based fluorescent assay for determination of ractopamine was developed with a dynamic range of 50 pM to 50 μM. The average recovery from spiked urine samples ranged from 95 to 110%, with an RSD of <1.5%.



Similar content being viewed by others
References
Zhou Y et al (2014) Sensitive immunoassay for the beta-agonist ractopamine based on glassy carbon electrode modified with gold nanoparticles and multi-walled carbon nanotubes in a film of poly-arginine. Microchim Acta 181(15–16):1973–1979
Chen S et al (2015) An on-site immunosensor for ractopamine based on a personal glucose meter and using magnetic beta-cyclodextrin-coated nanoparticles for enrichment, and an invertase-labeled nanogold probe for signal amplification. Microchim Acta 182(3–4):815–822
Zhang ZH et al (2015) Novel zinc oxide nanostructures fabrication by oxygen plasma surface modification and improvement of ractopamine detection. Plasma Chem Plasma Process 35(4):785–798
Zhang Z et al (2015) Manganese(II) phosphate nanoflowers as electrochemical biosensors for the high-sensitivity detection of ractopamine. Sensors Actuators B Chem 211:310–317
Du W et al (2014) Combined microextraction by packed sorbent and high-performance liquid chromatography-ultraviolet detection for rapid analysis of ractopamine in porcine muscle and urine samples. Food Chem 145:789–795
Mitchell GA, Dunnavan G (1998) Illegal use of beta-adrenergic agonists in the United States. J Anim Sci 76(1):208–211
Ding GL et al (2015) Development and validation of a high-performance liquid chromatography method for determination of ractopamine residue in pork samples by solid phase extraction and pre-column derivatization. Meat Sci 106:55–60
Vichapong J, Burakham R, Srijaranai S (2016) Determination of beta-agonists in porcine meats by ion-pair extraction and high performance liquid chromatography. Anal Lett 49(2):208–216
Gao HF et al (2014) Highly sensitive multianalyte immunochromatographic test strip for rapid chemiluminescent detection of ractopamine and salbutamol. Anal Chim Acta 839:91–96
Zhao L et al (2010) Simultaneous determination of melamine and clenbuterol in animal feeds by GC-MS. Chromatographia 72(3–4):365–368
Chen C et al (2015) Simultaneous separation and sensitive detection of four beta 2-agonists in biological specimens by CE-UV using a field-enhanced sample injection method. Anal Methods 7(1):175–180
Zhou Y et al (2013) Colorimetric detection of ractopamine and salbutamol using gold nanoparticles functionalized with melamine as a probe. Talanta 112:20–25
Li CY, Jiang JQ (2012) Preparation of a polyclonal antibody based heterologous indirect competitive ELISA for detecting ractopamine residue. Journal of Food Agriculture & Environment 10(2):1349–1352
Shelver WL, Smith DJ (2002) Application of a monoclonal antibody based ELISA for the determination of ractopamine in incurred samples from food animals. Abstr Pap Am Chem Soc 223:U56–U56
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249(4968):505–510
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346(6287):818–822
Jarujamrus P et al (2015) Selective colorimetric sensors based on the monitoring of an unmodified silver nanoparticles (AgNPs) reduction for a simple and rapid determination of mercury. Spectrochimica Acta Part a-Molecular and Biomolecular Spectroscopy 142:86–93
Lee SC et al (2015) Development of receptor-based inhibitory RNA aptamers for anthrax toxin neutralization. Int J Biol Macromol 77:293–302
Brockmann C et al (2015) Intravitreal inhibition of complement C5a reduces choroidal neovascularization in mice. Graefes Arch Clin Exp Ophthalmol 253(10):1695–1704
Romhildt L et al (2013) Patterned biochemical functionalization improves aptamer-based detection of unlabeled thrombin in a sandwich assay. ACS Appl Mater Interfaces 5(22):12029–12035
Eid C et al (2015) Rapid slow off-rate modified aptamer (SOMAmer)-based detection of C-reactive protein using Isotachophoresis and an ionic spacer. Anal Chem 87(13):6736–6743
Pang YF et al (2015) A fluorescent aptasensor for H5N1 influenza virus detection based-on the core-shell nanoparticles metal-enhanced fluorescence (MEF). Biosens Bioelectron 66:527–532
Abbaspour A et al (2015) Aptamer-conjugated silver nanoparticles for electrochemical dual-aptamer-based sandwich detection of Staphylococcus aureus. Biosens Bioelectron 68:149–155
Wu X et al (2015) DNA aptamer selected against pancreatic ductal adenocarcinoma for in vivo imaging and clinical tissue recognition. Theranostics 5(9):985–994
Golub E et al (2009) Electrochemical, Photoelectrochemical, and surface Plasmon resonance detection of cocaine using supramolecular aptamer complexes and metallic or semiconductor nanoparticles. Anal Chem 81(22):9291–9298
Liu J et al (2014) Highly sensitive colorimetric detection of 17beta-estradiol using split DNA aptamers immobilized on unmodified gold nanoparticles. Sci Rep 4:7571
Lv Z et al (2014) A simple and sensitive approach for ochratoxin a detection using a sensor fluorescent aptasensor. PLoS One 9(1):e85968
Yang F et al (2016) Label free electrochemical aptasensor for ultrasensitive detection of ractopamine. Biosens Bioelectron 77:347–352
Chen A, Yang S (2015) Replacing antibodies with aptamers in lateral flow immunoassay. Biosens Bioelectron 71:230–242
Noothi SK et al (2009) Enhanced DNA dynamics due to cationic reagents, topological states of dsDNA and high mobility group box 1 as probed by PicoGreen. FEBS J 276(2):541–551
Koba M, Szostek A, Konopa J (2007) Limitation of usage of PicoGreen dye in quantitative assays of double-stranded DNA in the presence of intercalating compounds. Acta Biochim Pol 54(4):883–886
Jenkins GR, Helber JT, Freese LD (2012) Concordance study: methods of quantifying corn and soybean genomic DNA intended for real-time polymerase chain reaction applications. J Agric Food Chem 60(34):8323–8332
Yu MX et al (2015) Rapid detection and enumeration of total bacteria in drinking water and tea beverages using a laboratory-built high-sensitivity flow cytometer. Anal Methods 7(7):3072–3079
Lv ZZ et al (2013) Highly sensitive fluorescent detection of small molecules, ions, and proteins using a universal label-free aptasensor. Chem Commun 49(48):5465–5467
Peterson EJR et al (2013) Inhibitors of Streptococcus Pneumoniae surface endonuclease EndA discovered by high-throughput screening using a PicoGreen fluorescence assay. J Biomol Screen 18(3):247–257
Wang KY et al (2015) A label-free aptasensor for highly sensitive detection of ATP and thrombin based on metal-enhanced PicoGreen fluorescence. Biosens Bioelectron 63:172–177
Wang P et al. (2016) An aptamer based assay for the β-adrenergic agonist ractopamine based on aggregation of gold nanoparticles in combination with a molecularly imprinted polymer. Microchimica Acta
Ren ML et al (2014) Lateral flow immunoassay for quantitative detection of ractopamine in swine urine. Biomed Environ Sci 27(2):134–137
Wang MY et al (2016) Enhanced simultaneous detection of ractopamine and salbutamol - via electrochemical-facial deposition of MnO2 nanoflowers onto 3D RGO/Ni foam templates. Biosens Bioelectron 78:259–266
Li X et al (2010) Development of rapid immunoassays for the detection of ractopamine in swine urine. Food Additives and Contaminants Part a-Chemistry Analysis Control Exposure & Risk Assessment 27(8):1096–1103
Dong JX et al (2012) Development of a single-chain variable fragment-alkaline phosphatase fusion protein and a sensitive direct competitive chemiluminescent enzyme immunoassay for detection of ractopamine in pork. Anal Chim Acta 736:85–91
Du W et al (2013) Combined solid-phase microextraction and high-performance liquid chromatography with ultroviolet detection for simultaneous analysis of clenbuterol, salbutamol and ractopamine in pig samples. Biomed Chromatogr 27(12):1775–1781
Acknowledgments
All authors gratefully acknowledge the funding support from the Special Fund for Agro Scientific Research in the Public Interest (201203046, 201203023).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 89 kb)
Rights and permissions
About this article
Cite this article
Zhu, C., Zhang, G., Huang, Y. et al. Aptamer based ultrasensitive determination of the β-adrenergic agonist ractopamine using PicoGreen as a fluorescent DNA probe. Microchim Acta 184, 439–444 (2017). https://doi.org/10.1007/s00604-016-2032-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-2032-z