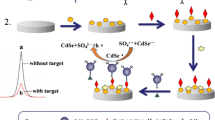
Abstract
A nanocomposite consisting of zinc sulfide quantum dots and polyaniline (ZnSQD@PANI) was placed on a gold electrode along with antibody against clenbuterol to give an amperometric immunosensor for clenbuterol. Compared to the use of pristine PANI, the electrode modified with the ZnSQD@PANI nanocomposite adsorbs clenbuterol antibody much better and therefore exhibits higher sensitivity to clenbuterol. The biosensor, when operated at a working potential of 0.21 V (vs. Ag/AgCl), displays a detection limit as low as 5.5 pg⋅mL−1 and works over the 0.01 to 10 ng⋅mL−1 concentration range. Related species such as salbutamol and ractopamine, urine components such as urea and uric acid, and the ions Ca(II), Na(I), and K(I) do not interfere.

A nanocomposite of zinc sulfide quantum dots and polyaniline was synthesized and utilized as the electrode material for detecting clenbuterol hydrochloride (CH). Sensing includes immunoglobulin G anchoring, adsorption of clenbuterol antibody, and detection of clenbuterol.







Similar content being viewed by others
References
Liu J, Zeng L, Li Z, Gao F, Huang X, Li F, Lin H (2009) Solid substrate-room temperature phosphorimetry for the determination of residual clenbuterol hydrochloride based on the catalysis of sodium periodate oxidizing eosine Y. Anal Chim Acta 638:69–74
Elliott C, McCaughey W, Shortt H (1993) Residues of the beta-agonist clenbuterol in tissues of medicated farm animals. Food Addit Contam 10:231–244
Sarmah AK, Meyer MT, Boxall AB (2006) A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65:725–759
Shao B, Jia X, Zhang J, Meng J, Wu Y, Duan H, Tu X (2009) Multi-residual analysis of 16 β-agonists in pig liver, kidney and muscle by ultra performance liquid chromatography tandem mass spectrometry. Food Chem 114:1115–1121
Liang WY, Ting Y, Hao SJ, Guo HW (2010) Determination of residual clenbuterol enantiomers in swine urine by high performance liquid chromatography. Chin J Anal Chem 38:833–837
García I, Sarabia L, Ortiz MC, Aldama JM (2004) Three-way models and detection capability of a gas chromatography–mass spectrometry method for the determination of clenbuterol in several biological matrices: the 2002/657/EC European decision. Anal Chim Acta 515:55–63
Zhou H, Zhang Z, He D, Hu Y, Huang Y, Chen D (2004) Flow chemiluminescence sensor for determination of clenbuterol based on molecularly imprinted polymer. Anal Chim Acta 523:237–242
He P, Shen L, Liu R, Luo Z, Li Z (2011) Direct detection of β-agonists by use of gold nanoparticle-based colorimetric assays. Anal Chem 83:6988–6995
Bai J, Lai Y, Jiang D, Zeng Y, Xian Y, Xiao F, Zhang N, Hou J, Jin L (2012) Ultrasensitive electrochemical immunoassay based on graphene oxide–Ag composites for rapid determination of clenbuterol. Analyst 137:4349–4355
Posyniak A, Zmudzki J, Niedzielska J (2003) Screening procedures for clenbuterol residue determination in bovine urine and liver matrices using enzyme-linked immunosorbent assay and liquid chromatography. Anal Chim Acta 483:61–67
Mitchell J (2010) Small molecule immunosensing using surface plasmon resonance. Sensors 10:7323–7346
Yan F, Zhang Y, Zhang ZJ, Liu S, He L, Feng X, Zhang H, Zhang Z (2015) Carboxyl-modified graphene for use in an immunoassay for the illegal feed additive clenbuterol using surface plasmon resonance and electrochemical impedance spectroscopy. Microchim Acta 182:855–862
Zhang L, Si HY, Zhang HL (2008) Highly ordered fluorescent rings by “breath figures” on patterned substrates using polymer-free CdSe quantum dots. J Mater Chem 18:2660–2665
Zrazhevskiy P, Sena M, Gao X (2010) Designing multifunctional quantum dots for bioimaging, detection, and drug delivery. Chem Soc Rev 39:4326–4354
Li Z, Wang Y, Wang J, Tang Z, Pounds JG, Lin Y (2010) Rapid and sensitive detection of protein biomarker using a portable fluorescence biosensor based on quantum dots and a lateral flow test strip. Anal Chem 82:7008–7014
Wang L, Qi W, Su R, He Z (2013) Facile method to synthesize graphene-ZnS nanocomposites: preparation and application in bioelectrochemistry of hemoglobin. J Solid State Electrochem 17:2595–2602
Barick K, Bahadur D (2010) Self-assembly of colloidal nanoscale particles: fabrication, properties and applications. J Nanosci Nanotechnol 10:668–689
Behrendt JM, Sutherland AJ (2009) Quantum dot-polymer bead composites for biological. Biosensing Using Nanomaterials 291
Pich A, Hain J, Lu Y, Boyko V, Prots Y, Adler H-J (2005) Hybrid microgels with ZnS inclusions. Macromolecules 38:6610–6619
Turner EA, Huang Y, Corrigan JF (2005) Synthetic routes to the encapsulation of II–VI semiconductors in mesoporous hosts. Eur J Inorg Chem 2005:4465–4478
Arya SK, Dey A, Bhansali S (2011) Polyaniline protected gold nanoparticles based mediator and label free electrochemical cortisol biosensor. Biosens Bioelectron 28:166–173
Li S, Xiong J, Shen J, Qin Y, Li J, Chu F, Kong Y, Deng L (2015) A novel hydrogen peroxide sensor based on Ag nanoparticles decorated polyaniline/graphene composites. J Appl Polym Sci 132
Dey SK, Sarkar D (2014) Effect of Zn source concentration on structural, optical and electrical properties of zinc sulphide–polyaniline (ZnS–PANI) nanocomposite thin films. J Mater Sci Mater Electron 25:5638–5645
Zhang L, Wei M, Gao C, Wei W, Zhang Y, Liu S (2015) Label-free electrochemical detection of methyltransferase activity and inhibitor screening based on endonuclease HpaII and the deposition of polyaniline. Biosens Bioelectron 73:188–194
Jablonska A, Gniadek M, Palys B (2015) Enhancement of direct electrocatalytic activity of horseradish peroxidase on polyaniline nanotubes. J Phys Chem C
Zhu Y, Liu E, Luo Z, Hu T, Liu T, Li Z, Zhao Q (2014) A hydroquinone redox electrolyte for polyaniline/SnO2 supercapacitors. Electrochim Acta 118:106–111
Garai A, Nandi AK (2009) Multiwalled carbon nanotube/polyaniline thermoreversible gel composites. Synth Met 159:1710–1716
Ayub A, Shakoor A, Elahi A, Rizvi TZ (2015) Optical and electronic properties of layer-by-layer and composite polyaniline-cadmium selenide quantum dot films. Superlattice Microst 84:154–164
Apte SK, Garaje SN, Arbuj SS, Kale BB, Baeg JO, Mulik UP, Naik SD, Amalnerkar DP, Gosavi SW (2011) A novel template free, one pot large scale synthesis of cubic zinc sulfide nanotriangles and its functionality as an efficient photocatalyst for hydrogen production and dye degradation. J Mater Chem 21:19241–19248
Wang J, Lim YF, Ho GW (2014) Carbon-ensemble-manipulated ZnS heterostructures for enhanced photocatalytic H2 evolution. Nanoscale 6:9673–9680
Fang S, Dong X, Zhang Y, Kang M, Liu S, Yan F, He L, Feng X, Wang P, Zhang Z (2014) One-step synthesis of porous cuprous oxide microspheres on reduced graphene oxide for selective detection of mercury ions. New J Chem
Miao P, Han K, Sun H, Yin J, Zhao J, Wang B, Tang Y (2014) Melamine functionalized silver nanoparticles as the probe for electrochemical sensing of clenbuterol. ACS Appl Mater Interfaces 6:8667–8672
Li Y, Qi P, Ma X, Zhong J (2014) Quick detection technique for clenbuterol hydrochloride by using surface plasmon resonance biosensor. Eur Food Res Technol 239:195–201
Gaichore RR, Srivastava AK (2012) Multiwalled carbon nanotube-4-tert-butyl calix [6] arene composite electrochemical sensor for clenbuterol hydrochloride determination by means of differential pulse adsorptive stripping voltammetry. J Appl Electrochem 42:979–987
Zhang X, Zhao H, Xue Y, Wu Z, Zhang Y, He Y, Li X, Yuan Z (2012) Colorimetric sensing of clenbuterol using gold nanoparticles in the presence of melamine. Biosens Bioelectron 34:112–117
Acknowledgments
This work was supported by Program for the National Natural Science Foundation of China (NSFC: Account No. 51173172) and Innovative Technology Team of Henan Province (2014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1192 kb)
Rights and permissions
About this article
Cite this article
Zhang, Z., Duan, F., He, L. et al. Electrochemical clenbuterol immunosensor based on a gold electrode modified with zinc sulfide quantum dots and polyaniline. Microchim Acta 183, 1089–1097 (2016). https://doi.org/10.1007/s00604-015-1730-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1730-2