Abstract
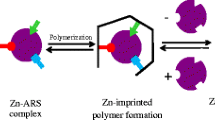
This article describes the preparation of a La(III) ion-imprinted nanoparticles (NPs) containing the La(III)-chelating ligand 2,2’:6’,6”-terpyridine (terpy). La(III) forms stable 1:1 and 1:2 complexes with terpy in acetonitrile solution. The NPs were prepared from ethyleneglycol dimethacrylate (the cross-linking monomer) via precipitation polymerization in the presence of the La(III)-terpy complex. La(III) ions were then removed from the NPs with nitric acid. The NPs were characterized by IR spectroscopy, scanning electron microscopy and elemental analysis. SEM micrographs showed the colloidal NPs to be slightly irregularly shaped and to have a diameter of 50 to 100 nm. The optimum pH value for sorption is 3.5. Sorption and desorption of La(III) is complete within 2 to 30 min. La(III) was quantified by ICP-AES. Figures of merit include a sorbent capacity for La(II) of 133.8 mg g−1, an enrichment factor of 17.5, a relative standard deviation of the determination of 1.7 % (at 3Sb/m), and a detection limit of 1.4 ng mL−1. The results indicate an increased affinity of the material toward La(III) over other multivalent metal ions of smaller ionic radius. The imprinted NPs were regenerated twenty times without a significant loss in affinity.

Ion-imprinted polymer nanoparticles were synthesized via precipitation polymerization and employed as a sorbent for the separation and preconcentration of trace amounts of lanthanum(III) ion from aqueous samples in the batch mode.





Similar content being viewed by others
References
Tokonami S, Shiigi H, Nagaoka T (2009) Review: micro- and nanosized molecularly imprinted polymers for high-throughput analytical applications. Anal Chim Acta 641:7–13
Downey JS, Frank RS, Li WH, Stöver HDH (1999) Growth mechanism of poly(divinylbenzene) microspheres in precipitation polymerization. Macromolecules 32:2838–2844
Naka Y, Yamamoto Y (1992) Preparation of microspheres by radiation-induced polymerization II. mechanism of microsphere growth. J Polym Sci Part A: Polym Chem 30:1287–1298
Chaitidou S, Kotrotsiou O, Kotti K, Kammona O, Bukhari M, Kiparissides C (2008) Precipitation polymerization for the synthesis of nanostructured particles. Mat Sci Eng B 152:55–59
Kala R, Gladis JM, Rao TP (2004) Preconcentrative separation of erbium from Y, Dy, Ho Tb and Tm by using ion imprinted polymer particles via solid phase extraction. Anal Chim Acta 518:143–150
Rao TP, Kala R, Daniel S (2006) Metal ion-imprinted polymers-novel materials for selective recognition of inorganics. Anal Chim Acta 578:105–116
Araki K, Marumaya T, Kamiya N, Goto M (2005) Metal ion-selective membrane prepared by surface molecular imprinting. J Chromatogr B 818:141–145
Shamsipur M, Besharati-Seidani A, Fasihi J, Sharghi H (2010) Synthesis and characterization of novel ion-imprinted polymeric nanoparticles for very fast and highly selective recognition of copper(II) ions. Talanta 83:674–681
Saatçılar Ö, Şatıroğlu N, Say R, Bektaş S, Denizli A (2006) Binding behaviour of Fe3+ ions on ion-imprinted polymeric beads for analytical applications. J Appl Polym Sci 101:3520–3528
Shamsipur M, Rajabi HZ, Beyzavi MH, Sharghi H (2013) Bulk polymer nanoparticles containing a tetrakis (3-hydroxyphenyl)porphyrin for fast and highly selective separation of mercury ions. Microchim Acta 180:791–799
Zambrzycka H, Godlewska-Żyłkiewicz B (2014) A new ion imprinted polymer based on Ru(III)-thiobarbituric acid complex for solid phase extraction of ruthenium(III) prior to its determination by ETAAS. Microchim Acta 181:1019–1027
Aliakbari A, Amini MM, Mehrani K, Moghadam Zadeh HR (2014) Magnetic ion imprinted polymer nanoparticles for the preconcentration of vanadium(IV) ions. Microchim Acta 181:1931–1938
Kala R, Rao TP (2006) Ion imprinted polymer particles for separation of yttrium from selected lanthanides. J Sep Sci 29:1281–1287
Bae SY, Southard GL, Murray GM (1999) Molecularly imprinted ion exchange resin for purification, preconcentration and determination of UO2 2+ by spectrophotometry and plasma spectrometry. Anal Chim Acta 397:173–181
El-Sofany EA (2008) Removal of lanthanum and gadolinium from nitrate medium using Aliquat-336 impregnated onto Amberlite XAD-4. J Hazard Mater 153:948–954
Saleh MI, Bari MF, Saad B (2002) Solvent extraction of lanthanum(III) from acidic nitrate acetate medium by Cyanex 272 in toluene. Hydrometallurgy 63:75–84
Fujimori E, Hayashi T, Inagaki K, Haraguchi H (1999) Determination of lanthanum and rare earth elements in bovine whole blood reference material by ICP-MS after coprecipitation preconcentration with heme-iron as coprecipitant Fresenius. J Anal Chem 363:277–282
Vizioli N, Gil R, Martínez LD, Silva MF (2009) On-line solid phase extraction CZE for the simultaneous determination of lanthanum and gadolinium at picogram per liter levels. Electrophoresis 30:2681–2687
De Jong N, Draye M, Favre-Réguillon A, LeBuzit G, Cote G, Foos J (2005) Lanthanum(III) and gadolinium(III) separation by cloud point extraction. J Colloid and Interf Sci 291:303–306
Tong S, Zhao S, Zhou W, Li R, Jia Q (2011) Modification of multi-walled carbon nanotubes with tannic acid for the adsorption of La, Tb and Lu ions. Microchim Acta 174:257–264
Rahman MM, Bahadar Khan S, Marwani HM, Asiri AM (2014) SnO2–TiO2 nanocomposites as new adsorbent for efficient removal of La(III) ions from aqueous solutions. J Taiwan Inst Chem Eng 45:1964–1974
Marwani HM, Alsafrani AE (2013) New solid phase extractor based on ionic liquid functionalized silica gel surface for selective separation and determination of lanthanum. J Anal Sci Technol 4:1–13
Sert S, Kütahyali C, İnan S, Talip Z, Çetinkaya B, Eral M (2008) Biosorption of lanthanum and cerium from aqueous solutions by Platanus orientalis leaf powder. Hydrometallurgy 90:13–18
Salhin A, Alhony AKM, Ab Ghani SB, Salleh B (2013) Acenaphthenequinone hydrazone derivative based Sol–gel in solid-phase extraction of lanthanum (III) in aqueous. World Appl Sci J 21:433–441
Wu D, Zhao J, Zhang L, Wu Q, Yang Y (2010) Lanthanum adsorption using iron oxide loaded calcium alginate beads. Hydrometallurgy 101:76–83
Dave SR, Kaur H, Menon SK (2010) Selective solid-phase extraction of rare earth elements by the chemically modified Amberlite XAD-4 resin with azacrown ether. React Funct Polym 70:692–698
Uezu K, Kuwabara T, Yoshida M, Goto M, Furusaki S (2004) Lanthanoid element recognition on surface-imprinted polymers containing dioleylphosphoric acid as a functional host. Anal Sci 20:1593–1597
Constable EC (1986) The coordination chemistry of 2,2’:6’,2”-terpyridine and higher oligopyridines. Adv Org Chem Radiochem 30:69–121
Dai H, Xiao D, He H, Li H, Yuan D, Zhang C (2014) Synthesis and analytical applications of molecularly imprinted polymers on the surface of carbon nanotubes: a review. Microchim Acta 1–16
Nicely VA, Dye JL (1971) A general purpose curve fitting program for class and research use. J Chem Educ 48:443–448
Büyüktiryaki S, Say R, Denizli A, Ersöz A (2007) Mimicking receptor for methylmercury preconcentration based on ion-imprinting. Talanta 71:699–705
Yoshida M, Uezu K, Goto M, Furusaki S (1999) Metal ion imprinted microsphere prepared by surface molecular imprinting technique using water-in-oil-in-water emulsions. J Appl Polym Sci 73:1223–1230
Labrou NE, Karagouni A, Clonis YD (1995) Biomimetic-dye affinity adsorbents for enzyme purification: application to the one-step purification of Candida boidinii formate dehydrogenase. Biotechnol Bioeng 48:278–288
Molochnikov LS, Kovalyova EG, Zagorodni AA, Muhammed M, Sultanov YM, Efendiev AA (2003) Coordination of Cu(II) and Ni(II) in polymers imprinted so as to optimize amine chelate formation. Polymer 44:4805–4815
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Besharati-Seidani, A., Shamsipur, M. Ion-imprinted polymeric nanoparticles for fast and selective separation of lanthanum(III). Microchim Acta 182, 1747–1755 (2015). https://doi.org/10.1007/s00604-015-1496-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1496-6