Abstract
The swelling of clay-sulfate rocks is a well-known phenomenon often causing threats to the success of various geotechnical projects, including tunneling, road and bridge construction, and geothermal drilling. The origin of clay-sulfate swelling is usually explained by physical swelling due to clay expansion combined with chemical swelling associated with the transformation of anhydrite (CaSO4) into gypsum (CaSO4∙2H2O). The latter occurs through anhydrite dissolution and subsequent gypsum precipitation. Numerical models that simulate rock swelling must consider hydraulic, mechanical, and chemical processes. The simulation of the chemical processes is performed by solving thermodynamic equations, which usually contribute a significant portion of the overall computation time. This paper employs feed-forward neural network (FFNN) and cascade-forward neural network (CFNN) models trained with a Bayesian regularization (BR) algorithm as an alternative approach to determine the solubility of anhydrite and gypsum in the aqueous phase. The network models are developed using calcium sulfate experimental data collected from the literature. Our results indicate that the FFNN-BR is the most accurate model for the regression task. The comparison analysis with the Pitzer ion interaction model as well as previously published data-driven models shows that the FFNN-BR model is highly accurate in determining the solubility of sulfate minerals in acid and salt-containing solutions. We conclude from our results that the FFNN-BR model can be used to determine the solubility of anhydrite and gypsum needed to address typical subsurface engineering problems such as swelling of clay-sulfate rocks.
Highlights
-
Neural network models have been developed to determine solubility of anhydrite and gypsum in multi-component electrolyte solutions.
-
An extensive solubility dataset has been employed to develop the neural network models.
-
Statistical analysis was performed on the solubility data obtained from intelligent and thermodynamic modeling approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The swelling of anhydrite-bearing clay rocks, also known as clay-sulfate rocks, has caused large problems in many geotechnical projects since more than 140 years ago with the construction of the Schanz railway tunnel in southern Germany (Schädlich et al. 2013). Despite advances in engineering methods and practical experiences gained during past decades, construction, e.g., tunneling, in swelling rocks is yet a challenging task because the accurate prediction of swelling pressures and deformations of clay-sulfate rocks is extremely difficult. Furthermore, ground heave, once the swelling is triggered by water inflow, may continue to develop for many years and it is practically impossible to stop the swelling (Schweizer et al. 2019).
The most remarkable problems with the swelling of clay-sulfate rocks are encountered during the construction of railway and road tunnels and viaducts. For example, some of the tunneling projects in Baden-Württemberg in Germany and Jura Mountains in Switzerland reported severe heave resulting from the uplift of materials below the tunnel floor (Anagnostou et al. 2010; Madsen and Müller-Vonmoos 1989; Wittke 2004; Wittke et al. 2007). In Spain, tunnels, e.g., Camp Magré, Lilla, and Puig Cabrer, constructed to provide a high-speed railway from Madrid to Barcelona, were affected by extreme sulfate-related heave (Alonso et al. 2013; Ramon et al. 2017).
A large-scale heave, causing catastrophic damage to buildings and roads, has taken place in the historic city of Staufen in southwest Germany, where geothermal drilling disturbed the hydrological system in the subsurface, allowing penetration of water into the clay-sulfate bearing formation of the Triassic Gipskeuper formation (“Gypsum Keuper”). Expansive hydration of the mineral anhydrite (CaSO4) led to substantial uplift of the ground and subsequently caused severe damages to infrastructures (Butscher et al. 2016, 2018; Jarzyna et al. 2021). Although the undertaken mitigation measures successfully decreased the uplift rate at the ground surface, they failed to completely stop the swelling process (Schweizer et al. 2019).
Swelling of clay-sulfate rocks may occur by clay swelling through osmotic water flow between surfaces of neighboring clay minerals, and sulfate swelling through the chemical transformation of anhydrite (CaSO4) into gypsum (CaSO4∙2H2O), which is the key process controlling the swelling phenomena (Butscher et al. 2016). Anhydrite dissolves in groundwater depending on temperature and ionic composition until the pore water becomes supersaturated with respect to gypsum. The dissolution of anhydrite then becomes accompanied by the crystallization of gypsum in particular when open spaces, e.g., fractures, are available. Gypsum crystals nucleate on existing gypsum crystals and surfaces of clay minerals. The transformation of anhydrite into gypsum proceeds until all the anhydrite present in the rock is converted to gypsum. This process (gypsification of anhydrite) is accompanied by about 61% increase in volume (Butscher et al. 2011, 2017; Jarzyna et al. 2021).
Since clay-sulfate rock swelling includes groundwater flow, mechanical swelling and chemical reaction, as well as their interaction, numerical models simulating the swelling should consider coupled thermo-hydro-mechanical-chemical processes (Butscher et al. 2016; Ramon et al. 2017). The calculation of chemical processes is usually performed by solving thermodynamic equations, which contributes a significant portion of the overall computational cost because compositions of liquid and mineral phases must be estimated at each iteration (e.g., Schweizer et al. 2018). Therefore, due to the high computational time, an efficient application of complex thermodynamic models, e.g., the Pitzer interaction model, in numerical simulation of coupled multi-physics processes in the subsurface environment is laborious (Hassanpouryouzband et al. 2021; Taherdangkoo et al. 2021).
As an alternative to complex thermodynamic models, artificial intelligence (AI) can be applied to determine the solubility of anhydrite and gypsum in the subsurface environment (Zarei et al. 2021). AI models do not require any pre-assumptions between input and output variables and are capable of handling complex nonlinear input–output relationships. However, AIs are subjected to some shortcomings, for instance, the stagnation in local minima during the training process and/or overfitting, which causes depictive outcomes. Optimization algorithms such as Bayesian Regularization have been proposed to overcome these issues and thus improve the performance of AI models (Benamara et al. 2020; Hassanpouryouzband et al. 2018; Taherdangkoo et al. 2020).
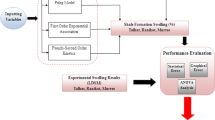
The primary goal of this study was to develop a robust neural network model able to determine the solubility of two calcium sulfates, gypsum and anhydrite, in aqueous solutions for further application in higher level numerical modeling. We used feed-forward and cascade-forward networks trained with a Bayesian regularization algorithm to perform the regression task. The network models were developed using an experimental dataset covering a wide range of conditions. A comparison analysis was designed to evaluate the performance of the most accurate network model with experimental data reported by Li and Demopoulos (2005, 2006), Marshall et al. (1964) and Marshall and Slusher (1966) as well as the Pitzer ion interaction model and two available data-driven models: the CMIS model (Rahmati et al. 2019) and the CSA-LSSVM model (Zarei et al. 2021).
2 Materials and Methods
2.1 Network Models
2.1.1 Feed-Forward Neural Network
Feed-forward neural network (FFNN) is a widely used neural network architecture. In a FFNN, information is processed in one direction, from input neurons, then through hidden neurons, and to output neurons. Therefore, the connections between the neurons do not form a cycle (Benamara et al. 2020; Svozil et al. 1997). The FFNNs are mainly employed for supervised learning tasks in cases where the input data are neither sequential nor time-dependent (Benardos and Vosniakos 2007; Lavine and Blank 2009).
2.1.2 Cascade-Forward Neural Network
Cascade-forward neural network (CFNN) is a specific type of ANNs recognized by its cascaded structure. Similar to the FFNN, the CFNN covers three categories of layers, namely input, hidden, and output layers (Nandagopal et al. 2017). However, the main specificity of CFNN is the cascade shape considered for linking neurons of different layers. This shape allows creating junctions between neurons of a given hidden layer with all the previous layers. This topology brings supplementary flexibility for dealing with complicated systems having larger numbers of input data. Besides, CFNNs have high degrees of generalization (Zeng et al. 2021; Zimmermann and Mattedi 2020).
2.1.3 Bayesian Regularization
Bayesian Regularization (BR) is a robust algorithm for optimizing weights and bias of various ANN types. BR can be considered as an improved version of the Levenberg–Marquardt algorithm as it involves the main principle of the latter with some modifications in the calculation procedure (Burden and Winkler 2009; MacKay 1992). In this context, the objective function aimed to be minimized is formulated to a weighted summation of squared network weights and squared errors. The coefficients of this function are determined by means of Bayes’ theorem (Burden and Winkler 2009; Foresee and Hagan 1997). The BR algorithm reduces the potential for overfitting while eliminating the need for the validation phase. Therefore, BR is especially suitable for small datasets (Taherdangkoo et al., 2020).
2.2 Existing Data-Driven Models
Rahmati et al. (2019) developed a committee machine intelligent system (CMIS) model to determine the solubility of gypsum in strong electrolyte solutions at temperatures ranging from 278.15 to 371.15 K. The CMIS model exhibited a reasonable predictive ability, showing good accordance with the experimental data. However, the CMIS model suffers from two deficiencies: (1) the model can only be applied to estimate the gypsum solubility, and (2) it is applicable for temperatures up to 371.15 K.
Recently, Zarei et al. (2021) developed a least-squares support vector machine tuned with coupled simulated annealing (CSA-LSSVM) to determine the solubility of gypsum, hemihydrate, and anhydrite in acid and salt-containing solutions at temperatures up to 373.15, 398.15, and 623.15 K, respectively. The model-associated deficiencies are (1) gypsum solubility in aqueous solutions can only be determined at temperatures up to 373.15 K, and therefore the applicability of the model in the geothermal field is limited. (2) the model fails to determine the solubility of calcium sulfates under some circumstances, for instance, it cannot accurately determine the solubility of gypsum in the system HCl + MgCl2 + CaCl2 + CaSO4 + H2O (Zarei et al. 2021).
2.3 Pitzer Model
The Pitzer ion interaction model has been widely used to describe the thermodynamic behavior of mixed electrolyte aqueous solutions. The Pitzer model is based on Debye–Hückel’s electrostatic theory for the long-range interactions and composition for the ion-specific short-range interactions in a solution. Harvie and Weare (1980) and Harvie et al. (1984) modified the Pitzer model by introducing asymmetrical electrostatic mixing terms into the model to improve the fit in multi-component systems. The mathematical expression and internal parameters of the model can be found in the relevant literature (Pitzer 1973; Pitzer and Mayorga 1973, 1974). The solubility products (Ksp) of the solid phases for calcium sulfates can be expressed as follows:
where γ and m are the activity coefficients of different species and molality, respectively. aw is the water activity. The activity coefficients of calcium and sulfate ions contributing to gypsum precipitation are calculated by the Pitzer model as follows:
where M and X are Ca2+ and SO42−, respectively. m is the molality, and I is the ionic strength, a and a' are anionic species, c and c' are cationic species, and Z equals \({\sum }_{i}{m}_{i}\left|{Z}_{i}\right|\). B and C represent the binary interaction parameters between ions of different signs. \(\Phi\) is the interaction between ions of the same sign, and \({\psi }_{ijk}\) is the ternary interaction parameter. Mw is the water molecular weight, and \(\varphi\) is the osmotic coefficient.
2.4 Data Acquisition
Experimental data of two calcium sulfates; gypsum (CaSO4·2H2O) and anhydrite (CaSO4) in aqueous electrolyte solutions were collected from the literature. The compiled solubility data were mostly reported in molality; therefore, the remaining data were converted to molality using appropriate conversion factors. The dataset contains a total of 1912 experimental data points (Azimi and Papangelakis 2010; Barba et al. 1984; Block and Waters 1968; Blount and Dickson 1973; Bock 1961; Furby et al. 1968; Hill and Wills 1938; Innorta et al. 1980; Kleinert and Wurm 1952; Kumar et al. 2005, 2004, 2007, 2010; Li and Demopoulos 2005, 2006; Ling and Demopoulos 2004; Madgin and Swales 1956; Marshall and Slusher 1966; Marshall et al. 1964; Ostroff and Metler 1966; Scheuermann et al. 2019; Wang et al. 2013; Wu et al. 2010; Yeatts and Marshall 1972; Yuan et al. 2010).
The dataset covers gypsum solubility in aqueous solutions containing NaCl, CaCl2, MgCl2, KCl, FeCl2, FeCl3, Na2SO4, HCl, AlCl3, MgSO4, H2SO4, and NaOH at temperatures ranging from 273.65 to 383.15 K, and anhydrite solubility in solutions containing H2SO4, NaCl, CaCl2, MgCl2, Na2SO4, and HCl at temperatures from 298 to 473.15 K. The statistical analysis and distribution of input and output data are summarized in Table 1 and Fig. 1, respectively. The calcium sulfate solubility (molality) is a function of temperature (K), and ionic composition of different salts (molality):
3 Results and Interpretation
3.1 Network Models
We used feed-forward and cascade-forward neural networks to determine calcium sulfate solubilities. Each model has one hidden layer having different numbers of neurons. Following the ratio of 80:20, the input dataset was randomly divided into training, and test sets. A sample of 1532 data was specified for the training phase, and the remaining data were used to analyze the reliability and robustness of the network models. The model performance was evaluated based on the mean squared error (MSE). The optimum network has the lowest MSE value without any noticeable difference between MSE values of training and test datasets, the opposite of which is an overfitting sign. The FFNN network trained with the BR algorithm, FFNN-BR model, has 26 neurons in the hidden layer, while the CFNN-BR has 12 neurons in the hidden layer.
Regression plots of predicted solubility values by FFNN-BR and CFNN-BR models versus measured solubility values depicted in Figs. 2 and 3 show that both models have yielded an acceptable accuracy, indicated by an accumulation of the data points close to the unit slope line. The statistical analysis listed in Table 2 shows that the FFNN-BR model has better performance compared to the CFNN-BR model. The FFNN-BR model has the MSE and R2 values of 5.67 × 10−5 and 0.97, respectively.
The quality of the FFNN-BR predictions is further illustrated in Fig. 4 with experimental and predicted values covering each other, indicating a prediction with the minimum error. The outputs of this model show a good agreement with the experimental values. The histogram plot of standardized residuals of the FFNN-BR model is depicted in Fig. 5. The residuals are mostly distributed near zero, indicating the outstanding performance of the model. Our statistical analysis indicates that the developed FFNN-BR is able to accurately determine solubility of gypsum and anhydrite in acid and salt-containing solutions in the ranges of temperature for which the model was trained.
3.2 Comparison Analysis
The predictions of the FFNN-BR model were compared with the experimental values of gypsum and anhydrite solubility in binary, ternary, and multi-component acid and salt-containing solutions reported by Li and Demopoulos (2005, 2006). The FFNN-BR model outputs were further compared with the Pitzer model as well as previously published data-driven models, including the CMIS model (Rahmati et al. 2019) and the CSA-LSSVM model (Zarei et al. 2021). Finally, the FFNN-BR was applied to determine experimental values of gypsum and anhydrite in aqueous sodium chloride solutions reported by Marshall et al. (1964) and Marshall and Slusher (1966). The designed comparison analysis provides an in-depth understanding on the behavior of the FFNN-BR model, and its overall performance to determine calcium sulfate hydrates solubilities in aquatic solutions.
Solubility of gypsum in HCl + CaCl2 + CaSO4 + H2O ternary system at three different temperatures, i.e., 295, 313, and 333 K, was measured by Li and Demopoulos (2006). The concentration of HCl is approximately 3 m. Figure 6 compares the experimental values with the predicted ones obtained from various models. The FFNN-BR outperforms the other models to determine gypsum solubility at 295 and 313 K. The results obtained from Pitzer and CMIS models are satisfactory. The CSA-LSSVM provides the least accurate predictions under these conditions. The Pitzer model is the most accurate to determine the gypsum solubility in this ternary system at 333 K. The FFNN-BR predictions are sufficiently accurate, following the expected trend.
Comparison of experimentally determined solubility of calcium sulfate as gypsum in the system HCl (3 mol/kg) + CaCl2 + CaSO4 + H2O at (a) 295, (b) 313, and (c) 333 K (Li and Demopoulos 2006) with the calculated values from Pitzer ion interaction and the FFNN-BR model of this study; and previously existing data-driven models CMIS (Rahmati et al. 2019) and CSA-LSSVM (Zarei et al. 2021) (colour figure online)
Solubility of gypsum in HCl + MgCl2 + CaSO4 + H2O ternary system at temperatures of 298, 323, and 353 K measured by Li and Demopoulos (2006) is compared with the predicted values, shown in Fig. 7. The concentration of HCl is approximately 3 m. The FFNN-BR and CMIS models are the most accurate in determining the solubility at 298 K. The CSA-LSSVM provides satisfactory performance. The FFNN-BR outperforms the other models at 323 K, while the CSA-LSSVM performs slightly better at 353 K. The Pitzer model shows the worst accuracy at predicting gypsum solubility in HCl + MgCl2 + CaSO4 + H2O ternary system, in particular at temperatures of 298 and 353 K.
Comparison of experimentally determined solubility of calcium sulfate as gypsum in the system HCl (3 mol/kg) + MgCl2 + CaSO4 + H2O at (a) 298, (b) 323, and (c) 353 K (Li and Demopoulos 2006) with the calculated values from Pitzer ion interaction and the FFNN-BR model of this study; and previously existing data-driven models CMIS (Rahmati et al. 2019) and CSA-LSSVM (Zarei et al. 2021) (colour figure online)
The FFNN-BR model is the most accurate model predicting gypsum solubility in the multi-component system of HCl + MgCl2 + CaCl2 + CaSO4 + H2O at 323 and 333 K (Fig. 8). The experimental data were measured by Li and Demopoulos (2006). The analysis shows that both Pitzer and CMIS models are sufficiently accurate. The CSA-LSSVM predictions deviate significantly from the experimental values, and thus the model predictions are not satisfactory.
Comparison of experimentally determined solubility of calcium sulfate as gypsum in the system HCl + MgCl2 (1 mol/kg) + CaCl2 + CaSO4 + H2O at (a) 323, and (b) 333 K (Li and Demopoulos 2006) with the calculated values from Pitzer ion interaction and the FFNN-BR model of this study; and previously existing data-driven models CMIS (Rahmati et al. 2019) and CSA-LSSVM (Zarei et al. 2021) (colour figure online)
The solubility of anhydrite in HCl + CaCl2 + CaSO4 + H2O system at temperatures of 298 and 313 K is measured by Li and Demopoulos (2005). Figure 9 shows that the Pitzer model is the most accurate model to determine the solubility values at 298 K. While the FFNN-BR model is highly accurate to determine the anhydrite solubilities at low CaCl2 concentrations (mol/kg ≤ 0.5), the model’s performance is lower at CaCl2 concentrations equal or above 1 m. Both FFNN-BR and CSA-LSSVM follow the trend observed in the experimental data. The FFNN-BR is the most accurate model to determine the solubility values at 313 K. The CSA-LSSVM predicted values are more accurate at 313 K as compared to the case of 298 K.
Comparison of experimentally determined solubility of calcium sulfate as anhydrite in the system HCl (3 mol/kg) + CaCl2 + CaSO4 + H2O system at (a) 298, and (b) 313 K (Li and Demopoulos 2005) with the calculated values from Pitzer ion interaction and the FFNN-BR model of this study; and previously existing data-driven model CSA-LSSVM (Zarei et al. 2021) (colour figure online)
The CSA-LSSVM is the most accurate model to determine the anhydrite solubility in HCl + CaSO4 + H2O system at the temperature of 298 and 313 K reported by Li and Demopoulos (2005) (Fig. 10). The Pitzer model performs better at 313 K compared to 298 K. Although the performance of the FFNN-BR model is lower than the other two models, the predicted values are sufficiently accurate. The FFNN-BR model performs better at low HCl concentrations.
Comparison of experimentally determined solubility of calcium sulfate as anhydrite in the system HCl + CaSO4 + H2O system at (a) 298, and (b) 313 K (Li and Demopoulos 2005) with the calculated values from Pitzer ion interaction and the FFNN-BR model of this study; and previously existing data-driven model CSA-LSSVM (Zarei et al. 2021) (colour figure online)
The FFNN-BR model exhibits excellent performance in predicting the solubility of anhydrite and gypsum in NaCl + CaSO4 + H2O system at 313.15, 353,15, and 448.15 K measured by Marshall et al. (1964) and Marshall and Slusher (1966) (Fig. 11). The model follows the expected trend observed in the experiments. For instance, the solubility of gypsum in aqueous NaCl solutions increases with the increase of NaCl concentrations and then experiences a decline (Fig. 11a). This behavior has been accurately captured by the FFNN-BR model.
4 Discussion
We follow from the presented results that the FFNN-BR model is able to determine the solubility of the calcium sulfates, gypsum and anhydrite, in complex acidic and electrolyte solutions with sufficient accuracy. The approach is, therefore, well suited for geological applications. In general, the model provides more accurate results as compared to the Pitzer model. In most cases studied, the FFNN-BR outperforms the CSA-LSSVM model, which is the only data-driven model that predicts the solubility of both gypsum and anhydrite in aqueous solutions. Similarly, in most cases, the predictions of the FFNN-BR are more accurate than the CMIS model, which is applicable for predicting the gypsum solubility. The FFNN-BR model is an accurate alternative to determine solubility of gypsum and anhydrite in mixed electrolyte solutions.
The FFNN-BR model can be further implemented in numerical modeling frameworks to address geotechnical problems such as swelling of clay-sulfate rocks. Numerical models that simulate rock swelling are usually coupled with a geochemical reaction model, e.g., PHREEQC (Charlton and Parkhurst 2011), to simulate the chemical processes. The FFNN-BR model is shown to be an effective tool to calculate solubility of calcium sulfates in mixed aquatic systems and is a promising alternative to coupling hydro-mechanical swelling models with geochemical reaction models such as PHREEQC. The FFNN-BR is more effective as compared to the Pitzer model, and has a large potential to reduce the overall computation run time required for modeling of complex THMC processes in the subsurface environment.
The FFNN-BR model should be applied under the ranges of conditions for which the model was trained. The application of the model outside of this specific domain is subjected to the modeler’s judgment to decide whether the prediction deviations are acceptable or not. Future studies may employ other data-driven models to predict solubility of calcium sulfate in aquatic systems. Other optimization algorithms such as metaheuristics algorithms can be tested to improve the performance of feed-forward neural network models. An accurate and sufficiently large dataset plays an important role in the development of data-driven models. Although a sufficiently large dataset is employed to develop the network models, future studies may enlarge the dataset to improve the accuracy and generalization ability of the model.
5 Summary and Conclusions
Modeling swelling behaviors of clay-sulfate rocks is complex as it involves complicated coupled thermo-hydro-mechanical-chemical processes. An accurate calcium sulfate solubility prediction framework is required for the numerical modeling of the chemical processes. This research employs feed-forward and cascade-forward neural network algorithms trained with Bayesian regularization to determine solubility of gypsum and anhydrite in aqueous electrolyte solutions. The compiled dataset includes experimental data covering a widespread range of temperature and salt concentrations. The FFNN-BR model is identified as the most accurate model for the task of calcium sulfate solubility prediction, yielding a R2 value of 0.966 and a MSE value of 0.0075. The FFNN-BR model presents an excellent performance in determining gypsum solubility. Although the model determines the anhydrite solubility with a slightly lower accuracy, the obtained values are sufficiently accurate. In general, the FFNN-BR model performs better compared to the Pitzer model and the other examined data-driven models. We conclude that the FFNN-BR model is a well-suited and robust tool which can be regarded as an alternative to more classical approaches for calcium sulfate solubility calculations. The proposed model can be further implemented in numerical modeling frameworks to effectively tackle complicated geotechnical problems, such as coupled THMC modeling of swelling processes of clay-sulfate rocks. This would reduce the required computation time and make numerical simulations more effective.
References
Alonso EE, Berdugo IR, Ramon A (2013) Extreme expansive phenomena in anhydritic-gypsiferous claystone: the case of Lilla tunnel. Géotechnique 63(7):584–612. https://doi.org/10.1680/geot.12.P.143
Anagnostou G, Pimentel E, Serafeimidis K (2010) Swelling of sulphatic claystones–some fundamental questions and their practical relevance. Geomech Tunn 3(5):567–572. https://doi.org/10.1002/geot.201000033
Azimi G, Papangelakis VG (2010) Thermodynamic modeling and experimental measurement of calcium sulfate in complex aqueous solutions. In: Fluid Phase Equilibria 290.1. Proceedings of the 17th Symposium on Thermophysical Properties. https://doi.org/10.1016/j.fluid.2009.09.023
Barba D, Brandani V, Di Giacomo G (1984) Solubility of calcium sulfate dihydrate in the system sodium sulfate-magnesium chloride-water. J Chem Eng Data 29(1):42–45. https://doi.org/10.1021/je00035a015
Benamara C, Gharbi K, Nait Amar M, Hamada B (2020) Prediction of wax appearance temperature using artificial intelligent techniques. Arab J Sci Eng 45(2):1319–1330. https://doi.org/10.1007/s13369-019-04290-y
Benardos PG, Vosniakos G-C (2007) Optimizing feedforward artificial neural network architec- ture. Eng Appl Artif Intell 20(3):365–382. https://doi.org/10.1016/j.engappai.2006.06.005
Block J, Waters OB (1968) Calcium sulfate-sodium sulfate-sodium chloride-water system at 25–100 ◦C. J Chem Eng Data 13(3):336–344. https://doi.org/10.1021/je60038a011
Blount CW, Dickson FW (1973) Gypsum-anhydrite equilibria in systems CaSO4∙H2O and CaCO4∙NaCl∙H2O. Am Mineral 58(3–4):323–331
Bock E (1961) On the solubility of anhydrous calcium sulphate and of gypsum in concentrated solutions of sodium chloride at 25 °C, 30 °C, 40 °C, and 50 °C. Can J Chem 39(9):1746–1751. https://doi.org/10.1139/v61-228
Burden F, Winkler D (2009) Bayesian regularization of neural networks. In: Livingstone DJ (ed) Artificial neural networks: methods and applications. Humana Press, Totowa, pp 23–42
Butscher C, Huggenberger P, Zechner E, Einstein HH (2011) Relation between hydrogeological setting and swelling potential of clay-sulfate rocks in tunneling. Eng Geol 122(3–4):204–214. https://doi.org/10.1016/j.enggeo.2011.05.009
Butscher C, Scheidler S, Farhadian H, Dresmann H, Huggenberger P (2017) Swelling potential of clay-sulfate rocks in tunneling in complex geological settings and impact of hydraulic measures assessed by 3D groundwater modeling. Eng Geol 221:143–153. https://doi.org/10.1016/j.enggeo.2017.03.010
Butscher C, Mutschler T, Blum P (2016) Swelling of clay-sulfate rocks: a review of processes and controls. Rock Mech Rock Eng 49(4):1533–1549. https://doi.org/10.1007/s00603-015-0827-6
Butscher C, Breuer S, Blum P (2018) Swelling laws for clay-sulfate rocks revisited. Bull Eng Geol Env 77:399–408. https://doi.org/10.1007/s10064-016-0986-z
Charlton SR, Parkhurst DL (2011) Modules based on the geochemical model PHREEQC for use in scripting and programming languages. Comput Geosci 37(10):1653–1663. https://doi.org/10.1016/j.cageo.2011.02.005
Foresee FD, Hagan MT (1997) Gauss-Newton approximation to Bayesian learning. In: Proceedings of International Conference on Neural Networks (ICNN’97), 1930–1935 vol. 3. https://doi.org/10.1109/ICNN.1997.614194.
Furby E, Glueckauf E, McDonald LA (1968) The solubility of calcium sulphate in sodium chloride and sea salt solutions. Desalination 4(2):264–276. https://doi.org/10.1016/S0011-9164(00)80290-8
Harvie CE, Weare JH (1980) The prediction of mineral solubilities in natural waters: the Na–K–Mg–Ca–SO4–Cl–H2O system from zero to high concentration at 25°C. Geochimica et Cosmochimica Acta 44(7):981–997. https://doi.org/10.1016/0016-7037(80)90287-2
Harvie CE, Møller N, Weare JH (1984) The prediction of mineral solubilities in natural waters: The Na–K–Mg–Ca–H–Cl–SO4–OH–HCO3–CO3–CO2–H2O system to high ionic strengths at 25 °C. Geochimica et Cosmochimica Acta 48(4):723–751. https://doi.org/10.1016/0016-7037(84)90098-X
Hassanpouryouzband A, Yang J, Tohidi B, Chuvilin E, Istomin V, Bukhanov B, Cheremisin A (2018) CO2 capture by injection of flue gas or CO2–N2 mixtures into hydrate reservoirs: dependence of CO2 capture efficiency on gas hydrate reservoir conditions. Environ Sci Technol 52(7):4324–4330. https://doi.org/10.1021/acs.est.7b05784
Hassanpouryouzband A, Joonaki E, Edlmann K, Haszeldine RS (2021) Offshore geological storage of hydrogen: is this our best option to achieve net-zero? ACS Energy Lett 6(6):2181–2186. https://doi.org/10.1021/acsenergylett.1c00845
Hill AE, Wills JH (1938) Ternary systems XXIV. Calcium sulfate, sodium sulfate and water. J Am Chem Soc 60(7):1647–1655. https://doi.org/10.1021/ja01274a037
Innorta G, Rabbi E, Tomadin L (1980) The gypsum-anhydrite equilibrium by solubility measurements. Geochimica et Cosmochimica Acta 44(12):1931–1936. https://doi.org/10.1016/0016-7037(80)90192-1
Jarzyna A, Bąbel M, Ługowski D, Vladi F (2021) Petrographic record and conditions of expansive hydration of anhydrite in the recent weathering zone at the abandoned dingwall gypsum quarry, Nova Scotia, Canada. Minerals 12(1):58. https://doi.org/10.3390/min12010058
Kleinert T, Wurm P (1952) Löslichkeitsuntersuchungen im wäßrigen System H2SO4- Na2SO4-CaSO4. Monatshefte für Chemie und verwandte Teile anderer Wissenschaften 83(2):459–462. https://doi.org/10.1007/BF00938571
Kumar A, Mohandas VP, Susarla VRKS, Ghosh PK (2004) Ionic interactions of calcium sulfate dihydrate in aqueous calcium chloride solutions: solubilities, densities, viscosities, and electrical conductivities at 30 °C. J Solut Chem 33(8):995–1003. https://doi.org/10.1023/B:JOSL.0000048049.62958.f9
Kumar A, Mohandas VP, Sanghavi R, Ghosh PK (2005) Ionic interactions of calcium sulfate dihydrate in aqueous sodium chloride solutions: solubilities, densities, viscosities, electrical conductivities, and surface tensions at 35C. J Solut Chem 34(3):333–342. https://doi.org/10.1007/s10953-005-3053-0
Kumar A, Sanghavi R, Mohandas VP (2007) Solubility pattern of CaSO4·2H2O in the system NaCl + CaCl2 + H2O and solution densities at 35 °C: non-ideality and ion pairing. J Chem Eng Data 52(3):902–905. https://doi.org/10.1021/je0604941
Kumar A, Shukla J, Dangar Y, Mohandas VP (2010) Effect of MgCl2 on the solubility of CaSO4·2H2O in the aqueous NaCl system and physicochemical solution properties at 35 °C. J Chem Eng Data 55(4):1675–1678. https://doi.org/10.1021/je900720y
Lavine BK, Blank TR (2009) Feed-forward neural networks. In: Brown SD, Tauler R, Walczak B (eds) Comprehensive chemometrics. Elsevier, Oxford, pp 571–586
Li Z, Demopoulos GP (2005) Solubility of CaSO4 phases in aqueous HCl + CaCl2 solutions from 283 K to 353 K. J Chem Eng Data 50(6):1971–1982. https://doi.org/10.1021/je050217e
Li Z, Demopoulos GP (2006) Effect of NaCl, MgCl2, FeCl2, FeCl3, and AlCl3 on solubility of CaSO4 phases in aqueous HCl or HCl + CaCl2 solutions at 298 to 353 K. J Chem Eng Data 51(2):569–576. https://doi.org/10.1021/je0504055
Ling Y, Demopoulos GP (2004) Solubility of calcium sulfate hydrates in (0 to 3.5) mol·kg-1 sulfuric acid solutions at 100 °C. J Chem Eng Data 49(5):1263–1268. https://doi.org/10.1021/je034238p
MacKay DJC (1992) A practical Bayesian framework for backpropagation networks. Neural Comput 4(3):448–472. https://doi.org/10.1162/neco.1992.4.3.448
Madgin WM, Swales DA (1956) Solubilities in the system CaSO4-NaCl-H2O at 25° and 35°. J Appl Chem 6(11):482–487. https://doi.org/10.1002/jctb.5010061102
Madsen FT, Müller-Vonmoos M (1989) The swelling behaviour of clays. Appl Clay Sci 4(2):143–156. https://doi.org/10.1016/0169-1317(89)90005-7
Marshall WL, Slusher R (1966) Thermodynamics of calcium sulfate dihydrate in aqueous sodium chloride solutions, 0–110°1,2. J Phys Chem 70(12):4015–4027. https://doi.org/10.1021/j100884a044
Marshall WL, Slusher R, Jones EV (1964) Aqueous systems at high temperatures XIV. Solubility and thermodynamic relationships for CaSO4 in NaCl-H2O solutions from 40 to 200°C., 0 to 4 Molal NaCl. J Chem Eng Data 9(2):187–191. https://doi.org/10.1021/je60021a011
Nandagopal MSG, Abraham E, Selvaraju N (2017) Advanced neural network prediction and system identification of liquid-liquid flow patterns in circular microchannels with varying angle of confluence. Chem Eng J 309:850–865. https://doi.org/10.1016/j.cej.2016.10.106
Ostroff AG, Metler AV (1966) Solubility of calcium sulfate dihydrate in the system NaCl-MgCl2-H2O from 28 to 70 °C. J Chem Eng Data 11(3):346–350. https://doi.org/10.1021/je60030a016
Pitzer KS (1973) Thermodynamics of electrolytes. I. Theoretical basis and general equations. J Phys Chem 77(2):268–277. https://doi.org/10.1021/j100621a026
Pitzer KS, Mayorga G (1973) Thermodynamics of electrolytes. II. Activity and osmotic coefficients for strong electrolytes with one or both ions univalent. J Phys Chem 77(19):2300–2308. https://doi.org/10.1021/j100638a009
Pitzer KS, Mayorga G (1974) Thermodynamics of electrolytes. III. Activity and osmotic coefficients for 2–2 electrolytes. J Solut Chem 3(7):539–546. https://doi.org/10.1007/BF00648138
Rahmati A, Gholamian M, Rostami S, Amirpour M, Safari H, Mohammadi AH (2019) An efficient model for estimation of gypsum (calcium sulfate di-hydrate) solubility in aqueous electrolyte solutions over wide temperature ranges. J Mol Liq 281:655–670. https://doi.org/10.1016/j.molliq.2019.02.077
Ramon A, Alonso EE, Olivella S (2017) Hydro-chemo-mechanical modelling of tunnels in sulfated rocks. Géotechnique 67(11):968–982. https://doi.org/10.1680/jgeot.SiP17.P.252
Schädlich B, Marcher T, Schweiger H (2013) Application of a constitutive model for swelling rock to tunnelling. Geotech Eng 44(3):47–54
Scheuermann PP, Tutolo BM, Seyfried Jr WE (2019) Anhydrite solubility in low-density hydrothermal fluids: experimental measurements and thermodynamic calculations. Chem Geol 524:184–195. https://doi.org/10.1016/j.chemgeo.2019.06.018
Schweizer D, Prommer H, Blum P, Siade AJ, Butscher C (2018) Reactive transport modeling of swelling processes in clay-sulfate rocks. Water Resour Res 54(9):6543–6565. https://doi.org/10.1029/2018WR023579
Schweizer D, Prommer H, Blum P, Butscher C (2019) Analyzing the heave of an entire city: modeling of swelling processes in clay-sulfate rocks. Eng Geol. https://doi.org/10.1016/j.enggeo.2019.105259
Svozil D, Kvasnicka V, Pospichal J (1997) Introduction to multi-layer feed-forward neural networks. Chemom Intell Lab Syst 39(1):43–62. https://doi.org/10.1016/S0169-7439(97)00061-0
Taherdangkoo R, Tatomir A, Taherdangkoo M, Qiu P, Sauter M (2020) Nonlinear autoregressive neural networks to predict hydraulic fracturing fluid leakage into shallow groundwater. Water. https://doi.org/10.3390/w12030841
Taherdangkoo R, Liu Q, Xing Y, Yang H, Cao V, Sauter M, Butscher C (2021) Predicting methane solubility in water and seawater by machine learning algorithms: application to methane transport modeling. J Contam Hydrol 242:103844. https://doi.org/10.1016/j.jconhyd.2021.103844
Wang W, Zeng D, Chen Q, Yin X (2013) Experimental determination and modeling of gypsum and insoluble anhydrite solubility in the system CaSO4-H2SO4-H2O. Chem Eng Sci 101:120–129. https://doi.org/10.1016/j.ces.2013.06.023
Wittke W (2007) New high-speed railway lines Stuttgart 21 and Wendlingen-Ulm: approximately 100 km of tunnels”. Underground Space—the 4th Dimension of Metropolises 1–3, p 771–778.
Wittke W, Wittke M, Wahlen R (2004) The source law of anhydrite containing Gipskeuper. Geotechnik 27(2):112–117
Wu X, He W, Guan B, Zhongbiao W (2010) Solubility of calcium sulfate dihydrate in Ca–Mg–K chloride salt solution in the range of (348.15 to 371.15) K. J Chem Eng Data 55(6):2100–2107. https://doi.org/10.1021/je900708d
Yeatts LB, Marshall WL (1972) Solubility of calcium sulfate dihydrate and association equilibriums in several aqueous mixed electrolyte salt systems at 25. deg. J Chem Eng Data 17(2):163–168. https://doi.org/10.1021/je60053a023
Yuan T, Wang J, Li Z (2010) Measurement and modelling of solubility for calcium sulfate dihydrate and calcium hydroxide in NaOH/KOH solutions. Fluid Phase Equilib 297(1):129–137. https://doi.org/10.1016/j.fluid.2010.06.012
Zarei MM, Hosseini M, Mohammadi AH, Moosavi A (2021) Model development for estimating calcium sulfate dihydrate, hemihydrate, and anhydrite solubilities in multicomponent acid and salt containing aqueous solutions over wide temperature ranges. J Mol Liq 328:115473. https://doi.org/10.1016/j.molliq.2021.115473
Zeng J, Jamei M, Amar MN, Hasanipanah M, Bayat P (2021) A novel solution for simulating air overpressure resulting from blasting using an efficient cascaded forward neural network. Eng Comput. https://doi.org/10.1007/s00366-021-01381-z
Zimmermann AS, Mattedi S (2020) Density and speed of sound prediction for binary mixtures of water and ammonium-based ionic liquids using feedforward and cascade forward neural networks. J Mol Liq 311:113212. https://doi.org/10.1016/j.molliq.2020.113212
Acknowledgements
We acknowledge the funding received from the German Research Foundation DFG for the project “coupled thermo-hydro-mechanical-chemical (THMC) processes in swelling clay-sulfate rocks (DFG BU 2993/2-2)”.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taherdangkoo, R., Meng, T., Amar, M.N. et al. Modeling Solubility of Anhydrite and Gypsum in Aqueous Solutions: Implications for Swelling of Clay-Sulfate Rocks. Rock Mech Rock Eng 55, 4391–4402 (2022). https://doi.org/10.1007/s00603-022-02872-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00603-022-02872-1