Abstract
Background
Variability in biological parameters may be associated with adverse outcomes. The aim of the study was to determine whether variability in body mass index (BMI) and blood pressure is associated with all-cause, cardiovascular mortality and cancer mortality or with renal disease progression in subjects with type 2 diabetes.
Methods
The diabetes database was accessed, and all the information on patient visits (consultations) carried out in the study period (1 January 2008–31 December 2019) was extracted and linked to the laboratory database and the mortality register.
Results
The total number of patients included in the study population was 26,261, of whom 54.4% were male. Median (interquartile range, IQR) age was 60.2 (51.8–68.3) years. The coefficient of variability of BMI was independently associated with increased all-cause and cardiovascular, but not cancer, mortality. Glycated haemoglobin (HbA1c) was associated with increased all-cause, cardiovascular, and cancer mortality as well as with renal progression. Variability in systolic blood pressure, diastolic blood pressure, and pulse pressure was associated with increased all-cause and cardiovascular mortality in bivariate, but not in multivariate, analyses.
Conclusions
Variability in BMI was associated with increased all-cause and cardiovascular, but not cancer, mortality in a large real-world contemporary population. Our results also confirm the association of HbA1c with increased all-cause, cardiovascular, and cancer mortality as well as with renal progression.
Similar content being viewed by others
Introduction
Variability in body mass index (BMI) has been associated with increased cardiovascular events and mortality in the general population [1,2,3,4], but this has not been confirmed by more recent studies [5,6,7,8]. Nam et al. [9] reported that in a Korean population with type 2 diabetes, body weight variability was associated with increased risks of MI, stroke, and all-cause mortality in patients with type 2 diabetes, but little data exist with respect to other racial groups. Recently, the Action for Health in Diabetes (Look AHEAD) trial in the US reported that fluctuations in BMI over a 4-year period were associated with adverse outcome in the control group but not in the intensive lifestyle intervention group [10]. Little data exist with regard to the relationship between fluctuations in BMI and cancer mortality or microvascular disease, but Chang et al. [11] recently reported that fluctuations in BMI were associated with increased risk of hepatocellular carcinoma in the general population, whilst a post hoc analysis of the ACCORD trial found that fluctuations in BMI in subjects with type 2 diabetes were associated with increased risk of renal events [12].
There are also little data on the effects of long-term blood pressure variability and outcomes in type 2 diabetes. Visit-to-visit variability in systolic blood pressure has been linked to increased all-cause mortality, coronary heart disease, stroke, and end-stage renal disease in the general population [13]. On the other hand, seasonal variability in blood pressure has been associated with better renal outcomes [14]. A retrospective study in China reported that visit-to-visit systolic blood pressure variability was a potential predictor for the development of cardiovascular and all-cause mortality in patients with type 2 diabetes [15].
The aim of the study was to determine whether variability in visit-to-visit BMI, systolic, diastolic blood pressure, and pulse pressure (as assessed by the coefficient of variation) is associated with (1) all-cause mortality; (2) cardiovascular mortality; (3) cancer mortality; (4) rise in albumin–creatinine ratio; and (5) progression of renal disease in subjects with type 2 diabetes.
Methodology
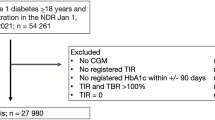
The Malta national diabetes database was accessed, and all the information on patient visits (consultations) carried out in the study period (1 January 2008–31 December 2019) was extracted. Exclusion criteria included: all patients who passed away before the start of the study period; any test results and visit data outside of the study period; and patients younger than 18 years of age at the start of the study period.
The extracted information included patient unique identifier (the national ID number), gender, date of birth, visit dates, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), and waist circumference. The national ID number was used to link the patients’ records to the National Mortality Registry—to extract the dates of death, and the underlying cause of death (using ICD10 coding). Causes of death were classified into cardiovascular (ICD-10 codes: I00-I09, I11, I13, I20-I51, I10, I12, I15, and I60-169) and cancer (ICD-10 codes: C00-C97, D00-D09, D10-D36, and D37-D48). It was also linked to the centralised Laboratory Information System so as to extract all the haemoglobin A1c (HbA1c) and urinary albumin–creatinine ratio (ACR) results of tests which were done during the study period.
The date of birth was used to calculate the age of patients at the start of the study period. The SBP and DBP were used to calculate the pulse pressure (PP). The median and coefficient of variation for the BMI, SBP, DBP, and PP were calculated for every patient where the number of observations per patient during the study period was more than one. This was calculated as standard deviation divided by the mean of all observations during the study period.
ACR results were categorised into three groups, namely normoalbuminuria: ACR < 30 mg/g; microalbuminuria: 30–300 mg/g; and macroalbuminuria: ACR > 300 mg/g. Progession of kidney disease was defined as transition from normoalbuminuria at baseline to microalbuminuria or to macroalbuminuria, or transition of micro-albuminuria to macroalbuminuria. Those with macroalbuminuria at baseline were excluded from these analyses as they cannot progress. Therefore, non-progessors included subjects who are normoalbuminuric at baseline and remained normoalbuminuric and those who were microalbuminuric at baseline and remained microalbuminuric or regressed to normoalbuminuria.
Statistical methods
Data tables were linked, and the data were analysed using R Studio running R version 4.2.3. Simple binary logistic regression was used for the continuous predictor variables and the Chi-square test for the factor predictor variables.
Multiple binary logistic regression was performed using backward elimination using a p value of < .05 as a cutoff to assess the relationship between the predictor and outcome variables: all-cause mortality, cardiovascular mortality, cancer mortality, and ACR progression which had a significant relationship in the bivariate analysis. Pulse pressure was not included in multivariate analyses since this would have violated one of the assumptions of multivariate analyses as the models also included systolic and diastolic blood pressure.
Results
The total number of patients included in the study population was: 26,261, of whom 54.4% were male. The median (interquartile range, IQR) age at baseline was 60.2 (51.8–68.3) years. The median (IQR) HbA1c was 7.10 (6.40–8.20) %, the median (IQR) BMI was 30.7 (27.3–34.6) whilst the median (IQR) waist circumference was 104 (96–112) cm. There was a median (IQR) of 11.0 (6.0–17.0) visits for every patient.
Tables 1, 2, and 3 show the results of bivariate analyses for all-cause, cardiovascular, and cancer mortality. Increasing age, higher HBA1c, ACR progression, higher systolic blood pressure, and higher pulse pressure were all associated with increased risk of all-cause and cardiovascular mortality. The coefficients of variation of BMI, systolic blood pressure, diastolic blood pressure, and pulse pressure were all associated with increased risk of all-cause and cardiovascular mortality. All-cause mortality was additionally associated with male gender and median ACR. Table 4 shows the results of multivariate analyses. Age was associated with increased all-cause mortality with an odds ratio (OR) (95% confidence interval, CI) of 1.14 (1.13–1.15); increased cardiovascular mortality with an OR (95% CI) of 1.11 (1.10–1.12); and increased cancer mortality with an OR (95% CI) of 1.06 (1.05–1.07) in multivariate analyses (p < .001 for all). HbA1c was also associated with increased all-cause, cardiovascular, and cancer mortality with OR (95% CI) of 1.51 (1.43–159), 1.51 (1.41–163), and 1.14 (1.08–1.20), respectively (p < .001 for all). Male gender was independently associated with increased all-cause and cancer mortality.
BMI variability, as assessed by the coefficient of variation, was associated with increased all-cause and cardiovascular mortality with OR (95% CI) of 14.6 (3.9–5.7, p < .001) and 7.37 (1.10–39.9, p = .028), respectively.
Only age, HbA1c, ACR, and pulse pressure were associated with ACR progression in bivariate analysis (Table 5). Age and HbA1c remained significant in multivariate analyses (Table 6).
Discussion
We found that variability of BMI, as assessed by the coefficient of variation, was associated with increased all-cause and cardiovascular, but not cancer, mortality in a population with Caucasian type 2 diabetes. This confirms what has been previously reported in a Korean population [9]. Our 10-year follow-up is the longest to report an association between fluctuation in BMI and cardiovascular outcome, whilst there are little data with respect to cancer mortality. Our results on fluctuating BMI are important because weight cycling is thought to be common, even in normal weight individuals [16] as a result of repeated attempts to lose weight and difficulty to maintain weight loss. Weight cycling is also associated with impaired psychological health [17]. There is evidence that weight loss beyond an individual’s set point is hindered by hormonal and metabolic changes [18]. It may, therefore, be important to set realistic targets for weight loss as this may be healthier than weight cycling, especially in individuals who have difficulty in maintaining weight loss.
Fluctuating BMI is due to repeated cycles of lipolysis and lipogenesis. Lipolysis results in increased circulating free fatty acids, which are known to be pro-inflammatory and to induce insulin resistance [19,20,21,22] and oxidative stress [23]. In fact, BMI cycling has been associated with hyperinsulinaemia, the metabolic syndrome, and higher pro-inflammatory markers [24,25,26]. The ‘repeated overshoot’ theory postulates that repeated overshoot of some cardiovascular factors during the weight regain phase of weight cycling may contribute to overall morbidity and mortality [16]. Weight cycling may also lead to preferential visceral fat accumulation [27], which is thought to be more detrimental. There is also evidence that weight gain following weight loss results in rapid expansion and hyperplasia of adipocytes [27], leading to an adverse adipokine profile. Variability in BMI was not independently associated with renal progression in our study, suggesting that the above mechanisms are less important in diabetic kidney disease.
Our results also confirm the association of HbA1c with increased all-cause, cardiovascular, and cancer mortality as well as with renal progression in a large real-world contemporary population. This is important since to date there are no convincing data that intensive glycaemic control improves survival. For example, a Cochrane review of 28 trials with a total 34,912 T2D participants found that intensive glycaemic control did not show significant differences for all-cause mortality and cardiovascular mortality control compared with conventional glycaemic control [28]. The reason for the failure of randomised controlled to find a beneficial effect of tight glycaemic control may be due to the short duration of many of them. In fact, the UK Prospective Study marginally missed statistical significance at 10 years [29], but demonstrated statistically lower all-cause mortality after a further 10-year observation period [30]. Most other trials were of shorter duration. Another reason could be the hypoglycaemia risk associated with some of the anti-hyperglcaemic agents used in many of these older studies.
We also report an association of HbA1c with cancer mortality. There are currently little data in the literature on the long-term effect of glycaemic control on cancer mortality, although a few recent studies have shown improved survival with better glycaemic control after diagnosis of some specific cancer types [31,32,33]. If confirmed by other authors, our findings may have important public health implications.
Our data show an association of variability in systolic blood pressure, diastolic blood pressure, and pulse pressure with increased all-cause and cardiovascular mortality in bivariate analyses. This is similar to the findings of earlier studies [34, 35] and to the results of post hoc pooled analysis of the Canagliflozin Cardiovascular Assessment Study (CANVAS) and Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) studies [36]. However, the relationship lost statistical significant in multivariate analysis in the present study. It should be noted that, unlike the previous studies, we also adjusted for variability in BMI in our multivariate analyses. This suggests that the previously reported association between variability in blood pressure and adverse cardiovascular outcome and all-cause mortality might be mediated through variability in BMI, given the known relationship between blood pressure and BMI [37]. This merits further study. Our data also show relationship between blood pressure variability and renal progression. However, this again lost statistical significance in multivariate analysis, suggesting that the association is mediated through the confounding effect of other parameters such as advancing age, which is known to be associated with higher blood pressure variability [38]. Our data are consistent with the pooled analysis of the CANVAS and CREDENCE studies referred to above, which also found that SBP variability was not associated with kidney outcomes [36].
In our study, baseline BMI was lower in non-survivors. We studied baseline rather than the mean BMI during the study period in order to minimise the possibility of reverse causality, namely that cardiac disease or malignancy leads to weight loss. The association of a higher BMI with lower cardiovascular [39,40,41,42] and cancer [43,44,45] mortality has been previously reported and is often referred to as the obesity paradox. The reasons for this are unclear, given the known association of adiposity with adverse outcomes [46]. However, the association lost statistical significance in multivariate analyses for all-cause and cardiovascular mortality in the present study. This suggests that the association between lower BMI and improved all-cause and cardiovascular survival is mediated by other factors such as decreasing BMI with advancing age. Reduction in BMI with advancing age is often due to due to loss of muscle, rather than fat, mass [47]. Loss of muscle mass can lead to insulin resistance [48], which is known to be associated with increased all-cause, cardiovascular disease [49,50,51] and cancer [51,52,53] mortality. Decreasing BMI may also be a marker of frailty, which is also known to be associated to increased mortality [54]. This is supported by the fact that we did not find an association of waist circumference with all-cause or cardiovascular mortality. The association remained significant with regard to cancer mortality in multivariate analysis. Possible explanations include weight loss as a result of occult malignancy or the presence of residual confounders such glycaemic control before the study period.
Strengths and limitations
One strength of our study is that we could access and link robust databases through the use of a unique identifier. These included the national diabetes electronic health record, the national health system’s laboratory information system, and the national mortality register. Other strengths include the large number of patients studied and the long follow-up period. Furthermore, the fact these databases included patients being cared for in primary care, secondary care, or both means that our results are more generalisable to the type 2 diabetes population. Nonetheless, our results need to be confirmed by other authors studying other populations and racial groups. Another limitation is that we do not have data on lifestyle factors such as smoking, diet, or exercise. Our study was not designed to study the relationship between BMI and blood pressure variability. Therefore, the possibility raised by our data that the association between blood pressure variability and adverse cardiovascular outcome and all-cause mortality may be mediated through variability in BMI needs confirmation in future appropriately designed studies.
Conclusions
Variability of BMI, as assessed by the coefficient of variation, was associated with increased all-cause and cardiovascular, but not cancer, mortality in a large real-world contemporary population. Our results also confirm the association of HbA1c with increased all-cause, cardiovascular, and cancer mortality as well as with renal progression. Furthermore, our results suggest that the previously reported association between variability in blood pressure and adverse cardiovascular outcome and all-cause mortality might be mediated through variability in BMI. Furthermore, our data provide further insights into the obesity paradox.
References
Kim DH, Nam GE, Han K, et al (2020) Variabilities in Weight and waist circumference and risk of myocardial infarction, stroke, and mortality: a nationwide Cohort study. Endocrinol Metab 35(4):933–942. https://doi.org/10.3803/EnM.2020.871
Lissner L, Odell PM, D’Agostino RB, et al (1991) Variability of body weight and health outcomes in the Framingham population. N Engl J Med 324:1839–1844. https://doi.org/10.1056/NEJM199106273242602
Blair SN, Shaten J, Brownell K, Collins G, Lissner L (1993) Body weight change, all-cause mortality, and cause-specific mortality in the multiple risk factor intervention trial. Ann Intern Med 119:749–757. https://doi.org/10.7326/0003-4819-119-7_part_2-199310011-00024
Peters ET, Seidell JC, Menotti A, et al (1995) Changes in body weight in relation to mortality in 6441 European middle-aged men: the Seven Countries Study. Int J Obes Relat Metab Disord 19:862–868
Roswall N, Li Y, Sandin S, Ström P, Adami HO, Weiderpass E (2017) Changes in body mass index and waist circumference and concurrent mortality among Swedish women. Obesity 25:215–222. https://doi.org/10.1002/oby.21675
Mousavi SV, Mohebi R, Mozaffary A, Sheikholeslami F, Azizi F, Hadaegh F (2015) Changes in body mass index, waist and hip circumferences, waist to hip ratio and risk of all-cause mortality in men. Eur J Clin Nutr 69:927–932. https://doi.org/10.1038/ejcn.2014.235
Field AE, Malspeis S, Willett WC (2009) Weight cycling and mortality among middle-aged or older women. Arch Intern Med 169:881–886. https://doi.org/10.1001/archinternmed.2009.67
Stevens VL, Jacobs EJ, Sun J, et al (2012) Weight cycling and mortality in a large prospective US study. Am J Epidemiol 175:785–792. https://doi.org/10.1093/aje/kwr378
Nam GE, Kim W, Han K, et al (2020) Body Weight variability and the risk of cardiovascular outcomes and mortality in patients with type 2 diabetes: a nationwide Cohort study. Diabetes Care 43:2234–2241. https://doi.org/10.2337/dc19-2552
Kaze AD, Santhanam P, Erqou S, Ahima RS, Bertoni AG, Echouffo-Tcheugui JB (2022) Body weight variability and risk of cardiovascular outcomes and death in the context of weight loss intervention among patients with type 2 diabetes. JAMA Netw Open 5(2):e220055. https://doi.org/10.1001/jamanetworkopen.2022.0055
Chang Y, Yoo JJ, Cho EJ, et al (2021) Weight fluctuation and risk of hepatocellular carcinoma: a nationwide population-based 8-million-subject study. Hepatol Int 15:482–492. https://doi.org/10.1007/s12072-021-10149-y
Hukportie DN, Li FR, Zhou R, Zheng JZ, Wu XX, Wu XB (2022) Waist circumference and body mass index variability and incident diabetic microvascular complications: a post hoc analysis of ACCORD trial. Diabetes Metab J. https://doi.org/10.4093/dmj.2021.0258
Gosmanova EO, Mikkelsen MK, Molnar MZ, et al (2016) Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, and renal disease. J Am Coll Cardiol 68:1375–1386. https://doi.org/10.1016/j.jacc.2016.06.054
See CY, Tseng CT, Lin WR, Chao JY, Kuo TH, Wang MC (2021) Seasonal change in home blood pressure monitoring is associated with renal outcome and mortality in patients with chronic kidney disease. Front Med 8:672651. https://doi.org/10.3389/fmed.2021.672651
Wan EY, Fung CS, Yu EY, Fong DY, Chen JY, Lam CL (2017) Association of visit-to-visit variability of systolic blood pressure with cardiovascular disease and mortality in primary care Chinese patients with type 2 diabetes-a retrospective population-based cohort study. Diabetes Care 40:270–279. https://doi.org/10.2337/dc16-1617
Montani JP, Schutz Y, Dulloo AG (2015) Dieting and weight cycling as risk factors for cardiometabolic diseases: who is really at risk? Obes Rev 16(Suppl 1):7–18. https://doi.org/10.1111/obr.12251
Petroni ML, Villanova N, Avagnina S, et al (2007) Psychological distress in morbid obesity in relation to weight history. Obes Surg 17:391–399. https://doi.org/10.1007/s11695-007-9069-3
Cicuttini FM, Proietto J, Lim YZ (2023) Our biology working against us in obesity: a narrative review on implications for management of osteoarthritis. Osteoarthr Cartil Open 5:100407. https://doi.org/10.1016/j.ocarto.2023.100407
Deng Q, Du L, Zhang Y, Liu G (2021) NEFAs influence the inflammatory and insulin signaling pathways through TLR4 in primary calf hepatocytes in vitro. Front Vet Sci 8:755505. https://doi.org/10.3389/fvets.2021.755505
Boden G (1997) Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 46:3–10
Capurso C, Capurso A (2012) From excess adiposity to insulin resistance: the role of free fatty acids. Vascul Pharmacol 57(2–4):91–97. https://doi.org/10.1016/j.vph.2012.05.003
Boden G (2008) Obesity and free fatty acids. Endocrinol Metab Clin North Am 37(3):635–646. https://doi.org/10.1016/j.ecl.2008.06.007
Steinberg HO, Tarshoby M, Monestel R, et al (1997) Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest 100:1230–1239
Shi X, Li D, Deng Q, et al (2015) NEFAs activate the oxidative stress-mediated NF-κB signaling pathway to induce inflammatory response in calf hepatocytes. J Steroid Biochem Mol Biol 145:103–112. https://doi.org/10.1016/j.jsbmb.2014.10.014
Yatsuya H, Tamakoshi K, Yoshida T, et al (2003) Association between weight fluctuation and fasting insulin concentration in Japanese men. Int J Obes Relat Metab Disord 27:478–483. https://doi.org/10.1038/sj.ijo.0802221
Zhang H, Tamakoshi K, Yatsuya H, et al (2005) Long-term body weight fluctuation is associated with metabolic syndrome independent of current body mass index among Japanese men. Circ J 69:13–18. https://doi.org/10.1253/circj.69.13
Tamakoshi K, Yatsuya H, Kondo T, et al (2003) Long-term body weight variability is associated with elevated C-reactive protein independent of current body mass index among Japanese men. Int J Obes Relat Metab Disord 27:1059–1065. https://doi.org/10.1038/sj.ijo.0802386
Cereda E, Malavazos AE, Caccialanza R, Rondanelli M, Fatati G, Barichella M (2011) Weight cycling is associated with body weight excess and abdominal fat accumulation: a cross-sectional study. Clin Nutr 30:718–723. https://doi.org/10.1016/j.clnu.2011.06.009
Hemmingsen B, Lund SS, Gluud C, et al (2013) Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD008143.pub3. (Update in: Cochrane Database Syst Rev. 2015;CD008143)
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352(9131):837–853. Erratum in: Lancet 1999 Aug 14;354(9178):602. PMID: 9742976
Stratton IM, Adler AI, Neil HA, et al (2000) Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321(7258):405–412. https://doi.org/10.1136/bmj.321.7258.405
Komatsu T, Chen-Yoshikawa TF, Ikeda M, Takahashi K, Nishimura A, Harashima SI et al (2020) Impact of diabetes mellitus on postoperative outcomes in individuals with non–small cell lung cancer: a retrospective cohort study. PLoS ONE 15:e0241930
Lee W, Yoon Y, Han H, Cho JY, Choi Y, Jang JY et al (2016) Prognostic relevance of preoperative diabetes mellitus and the degree of hyperglycemia on the outcomes of resected pancreatic ductal adenocarcinoma. J Surg Oncol 113:203–208
Wang NF, Tang HM, Liu FL, Hong QY (2020) Prolonged progression-free survival and overall survival are associated with diabetes mellitus but inversely associated with levels of blood glucose in patients with lung cancer. Chin Med J 133:786
Hata J, Arima H, Rothwell PM, et al (2013) Effects of visit-to-visit variability in systolic blood pressure on macrovascular and microvascular complications in patients with type 2 diabetes mellitus: the ADVANCE trial. Circulation 128:1325–1334. https://doi.org/10.1161/CIRCULATIONAHA.113.002717
Hsieh YT, Tu ST, Cho TJ, Chang SJ, Chen JF, Hsieh MC (2012) Visit-to-visit variability in blood pressure strongly predicts all-cause mortality in patients with type 2 diabetes: a 5·5-year prospective analysis. Eur J Clin Invest 42:245–253. https://doi.org/10.1111/j.1365-2362.2011.02574.x
Fletcher RA, Arnott C, Rockenschaub P, et al (2023) Canagliflozin, blood pressure variability, and risk of cardiovascular, kidney, and mortality outcomes: pooled individual participant data from the CANVAS and CREDENCE trials. J Am Heart Assoc 12:e028516. https://doi.org/10.1161/JAHA.122.028516
Jeoung B, Kim J (2023) A cross-sectional study of physical fitness and risk of hypertension in korean adults aged 20–59. Healthcare 11:2057. https://doi.org/10.3390/healthcare11142057
Bencivenga L, De Souto BP, Rolland Y, et al (2022) Blood pressure variability: a potential marker of aging. Ageing Res Rev 80:101677. https://doi.org/10.1016/j.arr.2022.101677
Dramé M, Godaert L (2023) The obesity paradox and mortality in older adults: a systematic review. Nutrients 15:1780. https://doi.org/10.3390/nu15071780
Akyea RK, Doehner W, Iyen B, Weng SF, Qureshi N, Ntaios G (2021) Obesity and long-term outcomes after incident stroke: a prospective population-based cohort study. J Cachexia Sarcopenia Muscle 12:2111–2121. https://doi.org/10.1002/jcsm.12818
Lempesis IG, Varrias D, Sagris M, et al (2023) Obesity and peripheral artery disease: current evidence and controversies. Curr Obes Rep 12:264–279. https://doi.org/10.1007/s13679-023-00510-7
Jones NR, Ordóñez-Mena JM, Roalfe AK, et al (2023) Body mass index and survival in people with heart failure. Heart. https://doi.org/10.1136/heartjnl-2023-322459
Li Y, Li C, Wu G, et al (2022) The obesity paradox in patients with colorectal cancer: a systematic review and meta-analysis. Nutr Rev 80:1755–1768. https://doi.org/10.1093/nutrit/nuac005
Kim LH, Doan P, He Y, Lau HM, Pleass H, Patel MI (2021) A systematic review and meta-analysis of the significance of body mass index on kidney cancer outcomes. J Urol 205:346–355. https://doi.org/10.1097/JU.0000000000001377
Greenlee H, Unger JM, LeBlanc M, Ramsey S, Hershman DL (2017) Association between body mass index and cancer survival in a pooled analysis of 22 clinical trials. Cancer Epidemiol Biomarkers Prev 26:21–29. https://doi.org/10.1158/1055-9965.EPI-15-1336
Parekh N, Lin Y, Hayes RB, Albu JB, Lu-Yao GL (2010) Longitudinal associations of blood markers of insulin and glucose metabolism and cancer mortality in the third National Health and Nutrition Examination Survey. Cancer Causes Control 21:631–642. https://doi.org/10.1007/s10552-009-9492-y
Shefflette A, Patel N, Caruso J (2023) Mitigating sarcopenia with diet and exercise. Int J Environ Res Public Health 20:6652. https://doi.org/10.3390/ijerph20176652.PMID:37681791;PMCID:PMC10487983
Seko T, Akasaka H, Koyama M, et al (2023) Preserved lower limb muscle mass prevents insulin resistance development in nondiabetic older adults. J Am Med Dir Assoc 24:376-381.e1. https://doi.org/10.1016/j.jamda.2022.12.002
Pyörälä M, Miettinen H, Laakso M, Pyörälä K (2000) Plasma insulin and all-cause, cardiovascular, and noncardiovascular mortality: the 22-year follow-up results of the Helsinki Policemen Study. Diabetes Care 23:1097–1102. https://doi.org/10.2337/diacare.23.8.1097
Hellgren MI, Daka B, Jansson PA, Lindblad U, Larsson CA (2015) Insulin resistance predicts early cardiovascular morbidity in men without diabetes mellitus, with effect modification by physical activity. Eur J Prev Cardiol 22:940–949. https://doi.org/10.1177/2047487314537917
Pan K, Chlebowski RT, Mortimer JE, et al (2020) Insulin resistance and breast cancer incidence and mortality in postmenopausal women in the Women’s Health Initiative. Cancer 126:3638–3647. https://doi.org/10.1002/cncr.33002
Sheng BW, Zhang JQ, Chen M, Ma M (2023) The inverse association between fasting blood glucose and the occurrence of gallbladder cancer in type 2 diabetes mellitus patients: a case-control study. J Cancer Res Clin Oncol 149:10387–10398. https://doi.org/10.1007/s00432-023-04944-3
Moshkovits Y, Rott D, Chetrit A, Dankner R (2022) The insulin sensitivity Mcauley index (MCAi) is associated with 40-year cancer mortality in a cohort of men and women free of diabetes at baseline. PLoS ONE 17:e0272437. https://doi.org/10.1371/journal.pone.0272437
Huang ST, Chen LK, Hsiao FY (2023) Clinical impacts of frailty on 123,172 people with diabetes mellitus considering the age of onset and drugs of choice: a nationwide population-based 10-year trajectory analysis. Age Ageing 52:afad128. https://doi.org/10.1093/ageing/afad128
Funding
Open Access funding provided by the University of Malta.
Author information
Authors and Affiliations
Contributions
SF conceived the study. SR performed the data extraction and analysis. Both authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. There was no external funding.
Ethical statement
The study was approved by the Research Ethics Committee of the University of Malta.
Informed consent
Not applicable.
Additional information
Managed by Massimo Federici.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fava, S., Reiff, S. Association of body mass index and blood pressure variability with 10-year mortality and renal disease progression in type 2 diabetes. Acta Diabetol (2024). https://doi.org/10.1007/s00592-024-02250-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00592-024-02250-z