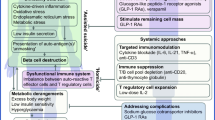
Abstract
In patients with type 1 diabetes (T1D), pancreatic β cells are destroyed by a selective autoimmune attack and their replacement with functional insulin-producing cells is the only possible cure for this disease. The field of islet transplantation has evolved significantly from the breakthrough of the Edmonton Protocol in 2000, since significant advances in islet isolation and engraftment, together with improved immunosuppressive strategies, have been reported. The main limitations, however, remain the insufficient supply of human tissue and the need for lifelong immunosuppression therapy. Great effort is then invested in finding innovative sources of insulin-producing β cells. One old alternative with new recent perspectives is the use of non-human donor cells, in particular porcine β cells. Also the field of preexisting β cell expansion has advanced, with the development of new human β cell lines. Yet, large-scale production of human insulin-producing cells from stem cells is the most recent and promising alternative. In particular, the optimization of in vitro strategies to differentiate human embryonic stem cells into mature insulin-secreting β cells has made considerable progress and recently led to the first clinical trial of stem cell treatment for T1D. Finally, the discovery that it is possible to derive human induced pluripotent stem cells from somatic cells has raised the possibility that a sufficient amount of patient-specific β cells can be derived from patients through cell reprogramming and differentiation, suggesting that in the future there might be a cell therapy without immunosuppression.

Similar content being viewed by others
References
Mannucci E, Monami M, Dicembrini I et al (2014) Achieving HbA1c targets in clinical trials and in the real world: a systematic review and meta-analysis. J Endocrinol Invest 37:477–495. doi:10.1007/s40618-014-0069-6
Van Belle TL, Coppieters KT, von Herrath MG (2011) Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 91:79–118. doi:10.1152/physrev.00003.2010
Lind M, Svensson A-M, Kosiborod M et al (2014) Glycemic control and excess mortality in type 1 diabetes. N Engl J Med 371:1972–1982. doi:10.1056/NEJMoa1408214
Saudek CD, Duckworth WC, Giobbie-Hurder A et al (1996) Implantable insulin pump vs multiple-dose insulin for non-insulin-dependent diabetes mellitus: a randomized clinical trial. Department of Veterans Affairs Implantable Insulin Pump Study Group. JAMA 276:1322–1327
Maffi P, Secchi A (2015) Clinical results of islet transplantation. Pharmacol Res 98:86–91. doi:10.1016/j.phrs.2015.04.010
Venturini M, Angeli E, Maffi P et al (2005) Technique, complications, and therapeutic efficacy of percutaneous transplantation of human pancreatic islet cells in type 1 diabetes: the role of US. Radiology 234:617–624. doi:10.1148/radiol.2342031356
Warnock GL, Kneteman NM, Ryan EA et al (1989) Continued function of pancreatic islets after transplantation in type I diabetes. Lancet 334:570–572. doi:10.1016/S0140-6736(89)90701-0
Scharp DW, Lacy PE, Santiago JV et al (1990) Insulin independence after islet transplantation into type I diabetic patient. Diabetes 39:515–518
Piemonti L, Pileggi A (2013) 25 Years of the Ricordi automated method for islet isolation. Cellr4 1(1):e128. http://www.cellr4.org/article/128. Accessed 29 Dec 2015
Bertuzzi F, Verzaro R, Provenzano V, Ricordi C (2007) Brittle type 1 diabetes mellitus. Curr Med Chem 14:1739–1744
Ballinger WF, Lacy PE (1972) Transplantation of intact pancreatic islets in rats. Surgery 72:175–186
Kemp CB, Knight MJ, Scharp DW et al (1973) Effect of transplantation site on the results of pancreatic islet isografts in diabetic rats. Diabetologia 9:486–491
Najarian JS, Sutherland DE, Matas AJ et al (1977) Human islet transplantation: a preliminary report. Transplant Proc 9:233–236
Ricordi C, Lacy PE, Finke EH et al (1988) Automated method for isolation of human pancreatic islets. Diabetes 37:413–420
Oberholzer J, Triponez F, Mage R et al (2000) Human islet transplantation: lessons from 13 autologous and 13 allogeneic transplantations. Transplantation 69:1115–1123
Secchi A, Socci C, Maffi P et al (1997) Islet transplantation in IDDM patients. Diabetologia 40:225–231
Shapiro AM, Lakey JR, Ryan EA et al (2000) Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 343:230–238. doi:10.1056/NEJM200007273430401
Shapiro AMJ, Ricordi C, Hering BJ et al (2006) International trial of the Edmonton protocol for islet transplantation. N Engl J Med 355:1318–1330. doi:10.1056/NEJMoa061267
Brennan DC, Kopetskie HA, Sayre PH et al (2015) Long-term follow-up of the edmonton protocol of islet transplantation in the United States. Am J Transplant. doi:10.1111/ajt.13458
Barton FB, Rickels MR, Alejandro R et al (2012) Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 35:1436–1445. doi:10.2337/dc12-0063
Brooks AM, Walker N, Aldibbiat A et al (2013) Attainment of metabolic goals in the integrated UK islet transplant program with locally isolated and transported preparations. Am J Transplant 13:3236–3243. doi:10.1111/ajt.12469
Vantyghem MC, Kerr-Conte J, Arnalsteen L et al (2009) Primary graft function, metabolic control, and graft survival after islet transplantation. Diabetes Care 32:1473–1478. doi:10.2337/dc08-1685
Lablanche S, Borot S, Wojtusciszyn A et al (2015) Five-year metabolic, functional, and safety results of patients with type 1 diabetes transplanted with allogenic islets within the Swiss-French GRAGIL network. Diabetes Care 38:1714–1722. doi:10.2337/dc15-0094
Qi M, Kinzer K, Danielson KK et al (2014) Five-year follow-up of patients with type 1 diabetes transplanted with allogeneic islets: the UIC experience. Acta Diabetol 51:833–843. doi:10.1007/s00592-014-0627-6
Bellin MD, Barton FB, Heitman A et al (2012) Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant 12:1576–1583. doi:10.1111/j.1600-6143.2011.03977.x
Shapiro AMJ (2011) State of the art of clinical islet transplantation and novel protocols of immunosuppression. Curr Diabetes Rep 11:345–354. doi:10.1007/s11892-011-0217-8
Posselt AM, Szot GL, Frassetto LA et al (2010) Islet transplantation in type 1 diabetic patients using calcineurin inhibitor-free immunosuppressive protocols based on T-cell adhesion or costimulation blockade. Transplantation 90(12):1595–1601
Nijhoff MF, Engelse MA, Dubbeld J et al (2015) Glycemic stability through islet-after-kidney transplantation using an alemtuzumab-based induction regimen and long-term triple-maintenance immunosuppression. Am J Transplant. doi:10.1111/ajt.13425
Maffi P, Berney T, Nano R et al (2014) Calcineurin inhibitor-free immunosuppressive regimen in type 1 diabetes patients receiving islet transplantation: single-group phase 1/2 trial. Transplantation. doi:10.1097/TP.0000000000000396
Posselt AM, Bellin MD, Tavakol M et al (2010) Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am J Transplant 10:1870–1880. doi:10.1111/j.1600-6143.2010.03073.x
El Khatib MM, Sakuma T, Tonne JM et al (2015) β-Cell-targeted blockage of PD1 and CTLA4 pathways prevents development of autoimmune diabetes and acute allogeneic islets rejection. Gene Ther 22:430–438. doi:10.1038/gt.2015.18
Watanabe M, Yamashita K, Suzuki T et al (2013) ASKP1240, a fully human anti-CD40 monoclonal antibody, prolongs pancreatic islet allograft survival in nonhuman primates. Am J Transplant 13:1976–1988. doi:10.1111/ajt.12330
Citro A, Cantarelli E, Maffi P et al (2012) CXCR1/2 inhibition enhances pancreatic islet survival after transplantation. J Clin Invest 122:3647–3651. doi:10.1172/JCI63089
Kandaswamy R, Skeans MA, Gustafson SK et al (2015) OPTN/SRTR 2013 annual data report: pancreas. Am J Transplant 15:1–20. doi:10.1111/ajt.13196
Klymiuk N, Aigner B, Brem G, Wolf E (2010) Genetic modification of pigs as organ donors for xenotransplantation. Mol Reprod Dev 77:209–221
Groth CG, Korsgren O, Tibell A et al (1994) Transplantation of porcine fetal pancreas to diabetic patients. Lancet (London, England) 344:1402–1404
Galili U, Shohet SB, Kobrin E et al (1988) Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem 263:17755–17762
Patience C, Takeuchi Y, Weiss RA (1997) Infection of human cells by an endogenous retrovirus of pigs. Nat Med 3:282–286
Cardona K, Korbutt GS, Milas Z et al (2006) Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med 12:304–306. doi:10.1038/nm1375
Hering BJ, Wijkstrom M, Graham ML et al (2006) Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med 12:301–303. doi:10.1038/nm1369
Shin JS, Kim JM, Kim JS et al (2015) Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am J Transplant 15:2837–2850. doi:10.1111/ajt.13345
Thompson P, Badell IR, Lowe M et al (2011) Islet xenotransplantation using gal-deficient neonatal donors improves engraftment and function. Am J Transplant 11:2593–2602. doi:10.1111/j.1600-6143.2011.03720.x
Bottino R, Wijkstrom M, van der Windt DJ et al (2014) Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. Am J Transplant 14:2275–2287. doi:10.1111/ajt.12868
Rayat GR, Rajotte RV, Ao Z, Korbutt GS (2000) Microencapsulation of neonatal porcine islets: protection from human antibody/complement-mediated cytolysis in vitro and long-term reversal of diabetes in nude mice. Transplantation 69:1084–1090
Dufrane D, Goebbels R-M, Gianello P (2010) Alginate macroencapsulation of pig islets allows correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression. Transplantation 90:1054–1062. doi:10.1097/TP.0b013e3181f6e267
Elliott RB, Escobar L, Tan PLJ et al (2007) Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation 14:157–161. doi:10.1111/j.1399-3089.2007.00384.x
Valdés-González RA, Dorantes LM, Garibay GN et al (2005) Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: a 4-year study. Eur J Endocrinol 153:419–427. doi:10.1530/eje.1.01982
Wang W, Mo Z, Ye B et al (2011) A clinical trial of xenotransplantation of neonatal pig islets for diabetic patients. Zhong Nan Da Xue Xue Bao Yi Xue Ban 36:1134–1140. doi:10.3969/j.issn.1672-7347.2011.12.002
Teta M, Long SY, Wartschow LM et al (2005) Very slow turnover of beta-cells in aged adult mice. Diabetes 54:2557–2567
Wang P, Fiaschi-Taesch NM, Vasavada RC et al (2015) Diabetes mellitus–advances and challenges in human β-cell proliferation. Nat Rev Endocrinol 11:201–212. doi:10.1038/nrendo.2015.9
Levine F, Wang S, Beattie GM et al (1995) Development of a cell line from the human fetal pancreas. Transplant Proc 27:3410
De la Tour D, Halvorsen T, Demeterco C et al (2001) Beta-cell differentiation from a human pancreatic cell line in vitro and in vivo. Mol Endocrinol 15:476–483
Narushima M, Kobayashi N, Okitsu T et al (2005) A human beta-cell line for transplantation therapy to control type 1 diabetes. Nat Biotechnol 23:1274–1282
Ravassard P, Hazhouz Y, Pechberty S et al (2011) A genetically engineered human pancreatic β cell line exhibiting glucose-inducible insulin secretion. J Clin Invest 121:3589–3597
Scharfmann R, Pechberty S, Hazhouz Y et al (2014) Development of a conditionally immortalized human pancreatic β cell line. J Clin Invest 124:2087–2098. doi:10.1172/JCI72674
Jones PM, Courtney ML, Burns CJ, Persaud SJ (2008) Cell-based treatments for diabetes. Drug Discov Today 13:888–893. doi:10.1016/j.drudis.2008.06.014
Thomson JA, Itskovitz-Eldor J, Shapiro SS et al (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147
D’Amour KA, Bang AG, Eliazer S et al (2006) Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24:1392–1401. doi:10.1038/nbt1259
Jiang W, Shi Y, Zhao D et al (2007) In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res 17:333–344. doi:10.1038/cr.2007.28
Jiang J, Au M, Lu K et al (2007) Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells 25:1940–1953. doi:10.1634/stemcells.2006-0761
Kroon E, Martinson LA, Kadoya K et al (2008) Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 26:443–452
Schulz TC, Young HY, Agulnick AD et al (2012) A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS ONE 7:e37004
Chen S, Borowiak M, Fox JL et al (2009) A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol 5:258–265. doi:10.1038/nchembio.154
Rezania A, Bruin JE, Xu J et al (2013) Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells 31:2432–2442. doi:10.1002/stem.1489
Nostro MC, Sarangi F, Yang C et al (2015) Efficient generation of NKX6-1+ pancreatic progenitors from multiple human pluripotent stem cell lines. Stem Cell Rep 4:591–604. doi:10.1016/j.stemcr.2015.02.017
Pagliuca FW, Millman JR, Gürtler M et al (2014) Generation of functional human pancreatic β cells in vitro. Cell 159:428–439. doi:10.1016/j.cell.2014.09.040
Rezania A, Bruin JE, Arora P et al (2014) Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. doi:10.1038/nbt.3033
Kelly OG, Chan MY, Martinson LA et al (2011) Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol 29:750–756
Jiang W, Sui X, Zhang D et al (2011) CD24: a novel surface marker for PDX1-positive pancreatic progenitors derived from human embryonic stem cells. Stem Cells 29:609–617
Osafune K, Caron L, Borowiak M et al (2008) Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol 26:313–315
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676. doi:10.1016/j.cell.2006.07.024
Yu J, Vodyanik MA, Smuga-Otto K et al (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920. doi:10.1126/science.1151526
Takahashi K, Tanabe K, Ohnuki M et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872. doi:10.1016/j.cell.2007.11.019
Tateishi K, He J, Taranova O et al (2008) Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem 283:31601–31607. doi:10.1074/jbc.M806597200
Schroeder IS, Rolletschek A, Blyszczuk P et al (2006) Differentiation of mouse embryonic stem cells to insulin-producing cells. Nat Protoc 1:495–507. doi:10.1038/nprot.2006.71
Alipio Z, Liao W, Roemer EJ et al (2010) Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic beta-like cells. Proc Natl Acad Sci USA 107:13426–13431. doi:10.1073/pnas.1007884107
Thatava T, Nelson TJ, Edukulla R et al (2011) Indolactam V/GLP-1-mediated differentiation of human iPS cells into glucose-responsive insulin-secreting progeny. Gene Ther 18:283–293. doi:10.1038/gt.2010.145
Zhang D, Jiang W, Liu M et al (2009) Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res 19:429–438. doi:10.1038/cr.2009.28
Kunisada Y, Tsubooka-Yamazoe N, Shoji M, Hosoya M (2012) Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res 8:274–284. doi:10.1016/j.scr.2011.10.002
Hua H, Shang L, Martinez H et al (2013) iPSC-derived β cells model diabetes due to glucokinase deficiency. J Clin Invest 123:3146–3153. doi:10.1172/JCI67638
Nostro MC, Sarangi F, Ogawa S et al (2011) Stage-specific signaling through TGFβ family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development 138:861–871. doi:10.1242/dev.055236
Pellegrini S, Ungaro F, Mercalli A et al (2015) Human induced pluripotent stem cells differentiate into insulin-producing cells able to engraft in vivo. Acta Diabetol 52:1025–1035. doi:10.1007/s00592-015-0726-z
Maehr R, Chen S, Snitow M et al (2009) Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci USA 106:15768–15773. doi:10.1073/pnas.0906894106
Nakagawa M, Koyanagi M, Tanabe K et al (2008) Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 26:101–106. doi:10.1038/nbt1374
Singh VK, Kalsan M, Kumar N et al (2015) Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. doi:10.3389/fcell.2015.00002
Motté E, Szepessy E, Suenens K et al (2014) Composition and function of macroencapsulated human embryonic stem cell-derived implants: comparison with clinical human islet cell grafts. Am J Physiol Endocrinol Metab 307:E838–E846. doi:10.1152/ajpendo.00219.2014
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All works cited in this review have been published in journals that require approval by the Ethics Committees of the conducted experiments.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Informed consent
All works cited in this review have been published in journals that require that informed consents of participants to reported clinical trials are collected.
Additional information
Managed by Massimo Federici.
Rights and permissions
About this article
Cite this article
Pellegrini, S., Cantarelli, E., Sordi, V. et al. The state of the art of islet transplantation and cell therapy in type 1 diabetes. Acta Diabetol 53, 683–691 (2016). https://doi.org/10.1007/s00592-016-0847-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-016-0847-z