Abstract
Purpose
The purpose of the study was to assess the changes in flexibility during night-time bracing in skeletally immature adolescent idiopathic scoliosis (AIS) with curves in the surgical range.
Materials and methods
We included a consecutive cohort of 89 AIS patients with curves ≥ 45° and an estimated growth potential. All patients were eventually treated with fusion surgery, and all patients had side-bending radiographs prior to both bracing and surgery. Curves were classified as structural or non-structural curves according to Lenke at both timepoints.
Results
The main curve progressed by a mean of 12 ± 10° and the secondary curve by 8 ± 8°. Flexibility of the main curve decreased from 50 ± 19% to 44 ± 19% (p = 0.001) and the underlying curve from 85 ± 21% to 77 ± 22% (p = 0.005). In 69 patients (79%), the Lenke category did not progress during bracing. In 14 patients (15%), the progression in Lenke type occurred in the thoracic region (i.e., Lenke type 1 to type 2), while six patients (7%) progressed in the lumbar region (i.e., type 1 to type 3).
In the 69 patients that did not progress, we found that the last touched vertebra moved distally by one or two levels in 26 patients.
Conclusions
This is the first study to describe that curve flexibility decreases during bracing in severe AIS. However, this had only a modest impact on the surgical strategy. Bracing as a holding strategy can be applied, but the risk of losing flexibility in the lumbar spine should be outweighed against the risks of premature fusion surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bracing in adolescent idiopathic scoliosis (AIS) is a well-established treatment modality [1]. Traditional bracing indications are skeletally immature patients with curves between 25° and 40° [2, 3]. The effect of bracing in larger curves is questionable, although there are reports of efficacy in curves more than 40° [4, 5]. Surgical management of AIS may be indicated when curves exceed 45° or 50° [1, 2]. Instrumented fusion has shown satisfactory results in terms of curve correction and achieving a stable solid fusion. However, the decision to perform fusion surgery in skeletally immature patients carries inherent risks, including the development of the crankshaft phenomenon, distal adding-on, and progression in the uninstrumented curves [6,7,8]. Ideally, fusion surgery should be avoided in skeletally immature patients, but the consequences of a conservative treatment approach in this high-risk period are unknown. The current literature does not guide the appropriate management of skeletally immature AIS patients with curves in the surgical range. In early-onset scoliosis, the principle of casting and bracing as a delay tactic prior to surgery is established [9] but is not well described in AIS patients. The theoretical risks involved with bracing are further curve progression, a larger extend of the main curve, and progression of flexible secondary curves to stiff, structural curves that require fusion.
The aim of the study was to assess the changes in flexibility during night-time bracing in skeletally immature patients with curves in the surgical range.
Materials and methods
This was a retrospective study on a consecutive cohort of AIS patients treated with a night-time Providence brace from 2008 through 2017. Inclusion criteria were curves ≥ 45° and an estimated growth potential. Estimation of growth potential was not standardized. As these were all progressive curves, generally, patients would be advised to brace if there was a suspected growth potential based on either Risser grading, menarchal status, Sanders stage, or observed continued height growth.
We included only patients who were eventually treated with fusion surgery. Exclusion criteria were syndromic or congenital scoliosis, prior brace treatment or failure to comply with brace treatment. We reviewed the medical chart to verify the diagnosis and compliance with treatment.
We analyzed coronal and sagittal radiographs at brace initiation and before surgery. All patients had prone side-bending radiographs prior to both bracing and surgery. From this, we calculated flexibility of the main curve and for the overlying and underlying if applicable. We applied the criteria for structural curves as defined by Lenke and categorized patients in Lenke type before and after bracing [10].
For each coronal radiograph, we defined the lower-end vertebra of the main curve, the neutral vertebra, and the last touched vertebra (LTV). LTV was defined as the most cranial vertebra touched by the central sacral vertical line.
Statistical analysis
The primary outcome was progression in Lenke category, requiring a more extensive fusion selection after bracing. Data were reported as proportions (%), mean with standard deviation, or median with interquartile range. For the univariate comparative analysis, continuous data were compared between groups using unpaired, two-tailed t test, or Wilcoxon rank sum test. Categorical variables were compared using Pearson’s chi-squared test. We conducted a post-hoc analysis on patients that progressed in curve type by univariate analysis on age, curve size, and curve type as this was hypothesized to predict progression. The significance level was set at 0.05.
Results
A total of 89 patients were included. Mean age at brace initiation was 13.4 ± 1.5 years, and median time in brace was 19 months (IQR: 13–19) (Table 1). The main curve progressed by a mean of 12 ± 10° and the lumbar curve by 8 ± 8°. Flexibility of the main curve decreased from 50 ± 19% to 44 ± 19% (p = 0.001) and the underlying curve from 85 ± 21% to 77 ± 22% (p = 0.005). In 69 patients (78%), the Lenke type did not progress during bracing (Figs. 1 and 2). In 14 patients (15%), the progression in Lenke type occurred in the thoracic region (i.e., Lenke type 1 to type 2), while six patients (7%) progressed in the lumbar region (i.e., type 1 to type 3) (Fig. 3). Mean age in the progression group was 12.7 years (SD: 1.6) vs. 13.6 ± 1.4 in the non-progression group (p = 0.021) (Table 2). There were no significant differences between progression and non-progression groups in terms of curve size, curve type, and curve flexibility at the start of brace treatment (Table 2).
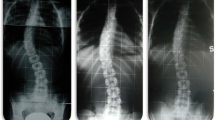
A 12-year-old patient with a Lenke 1C curve before night-time bracing was initiated. The thoracic curve is 54°, and the lumbar curve is 45°. At follow-up before surgery, the thoracic curve has progressed to 75°, but the end vertebra has not changed. The lumbar curve is unchanged and remains flexible
In the 69 patients that did not progress, we found that the last touched vertebra moved distally by one level in 21 (30%) patients and by two levels in five cases (7%) (Fig. 4).
Discussion
The concept of bracing as a holding strategy in large AIS curves for patients with substantial growth potential remaining has not been well-documented in the literature. This study addresses this gap by retrospectively analyzing a cohort of AIS patients with severe curves treated with night-time bracing. We found curve progression in both the main and secondary curves, and that curves became less flexible during bracing. However, in the vast majority of patients (79%), the Lenke curve type did not progress during bracing. Notably, in those who did progress, the majority progressed in the proximal thoracic which was expected, since bracing of the proximal thoracic curve is challenging [11]. Also, this region is of less concern since fusion of the proximal curve is not likely to significantly affect the patient’s quality of life. Looking at the lumbar curve, six patients (7%) progressed from a non-structural to a structural curve, which would typically mean an indication for fusion of the lumbar curve. Fusion of the lumbar curve can result in early lumbar degenerative changes and decreased patient satisfaction [12]. In our study, age at brace initiation was identified as a significant factor associated with progression, with younger patients at a slightly higher risk (p = 0.01). However, there were no significant differences in terms of curve size, curve type, or curve flexibility at the start of brace treatment between the progression and non-progression groups. Our study does not assess the optimal time for surgical intervention. Historical data have suggested that crankshaft phenomenon can be avoided by waiting for closure of the triradiate cartilage [13, 14], while distal adding-on seems to occur significantly more frequent in Risser grade 2 or less at the time of surgery [6].
This is the first study to report on changes in flexibility during bracing, and as such, there are no comparable data in the literature. Vertebral body tethering may provide an alternative to bracing for treating skeletally immature scoliosis patient, either as a stand-alone procedure or as a hybrid (lumbar tethering and thoracic fusion) [15,16,17]. The available data suggest that the lumbar unfused curve also corrects in most cases [16, 18]. While these techniques have shown good results in carefully selected patients, they require a substantial amount of growth potential and are not suitable for patients who have completed the growth spurt [19]. Bracing could be a better alternative in these patients. To our knowledge, no study has examined curve flexibility before and after tethering, and a complication rate of more than 20% and low efficacy in moderate skeletal immaturity should be taken into consideration [20]. Physiotherapeutic scoliosis-specific exercises in combination with bracing have gained popularity in some centers, but whether this can limit curve progression and maintain flexibility in severe curves is unknown [21].
In the group of patients who did not progress with regard to Lenke type, a subset (38%) exhibited changes in the LTV, which moved distally by one or two levels. The LTV has gained increased focus as a suitable selection for the lowest instrumented vertebra in selective thoracic fusion [22,23,24]. As such, the distalization of the LTV has the potential implication of an added final fusion by one or two levels. In the lower lumbar area, this can have substantial impact on the surgical outcome [25, 26], while the available data do not show a deleterious effect of an added fusion level in the lower thoracic/upper lumbar area [27]. However, the risk of adding-on is increased from 12% to 19% (Risser stages 0–5) and 13% to 43% in patients with open versus closed triradiate cartilage [28]. These considerations may favor bracing as a holding strategy until relative skeletal maturity. While this study provides insights into the potential benefits of night-time bracing in skeletally immature AIS patients with surgical-range curves, several limitations should be considered. The main limitation is the lack of a control group. We cannot address the fundamental question of whether the brace treatment changed the natural course of the severe deformity. Also, this study focused on flexibility changes during bracing, not on efficacy of bracing in terms of preventing curve progression. Patients were only included if they had undergone surgical treatment (including a second set of flexibility radiographs), while patients with large curves that decided not to undergo surgical treatment were not included. Also, the indications for bracing were not standardized, and maturity assessment was based on a variety of factors. This may influence the external validity, although we consider our approach to reflect real-life clinical practice.
Conclusion
This is the first study to describe that curve flexibility decreases during bracing in severe AIS. However, in our cohort, this rarely had a substantial impact on the surgical strategy. We regard bracing as a holding strategy that can be applied, and the risk of losing flexibility in the lumbar spine is outweighed against the risks of premature fusion surgery.
References
Weinstein SL, Dolan LA, Wright JG, Dobbs MB (2013) Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med 369:1512–21. https://doi.org/10.1056/NEJMoa1307337
Negrini S, Hresko TM, O’Brien JP, Price N (2015) Recommendations for research studies on treatment of idiopathic scoliosis: consensus 2014 between SOSORT and SRS non–operative management committee. Scoliosis 10:1–12
Richards BS, Bernstein RM, D’Amato CR, Thompson GH (2005) Standardization of criteria for adolescent idiopathic scoliosis brace studies: SRS Committee on Bracing and Nonoperative Management. Spine (Phila Pa 1976) 30:2068–75
Aulisa AG, Guzzanti V, Falciglia F et al (2019) Brace treatment of Idiopathic Scoliosis is effective for a curve over 40 degrees, but is the evaluation of Cobb angle the only parameter for the indication of treatment? Eur J Phys Rehabil Med 55. https://doi.org/10.23736/S1973-9087.18.04782-2
Zhang T, Huang Z, Sui W et al (2023) Intensive bracing management combined with physiotherapeutic scoliosis-specific exercises for adolescent idiopathic scoliosis patients with a major curve ranging from 40–60° who refused surgery: a prospective cohort study. Eur J Phys Rehabil Med 59. https://doi.org/10.23736/S1973-9087.23.07605-0
Ohrt-Nissen S, Luk KDK, Samartzis D, Cheung JPY (2020) Selection of the lowest instrumented vertebra in main thoracic adolescent idiopathic scoliosis: is it safe to fuse shorter than the last touched vertebra? Eur Spine J 29:2018–2024. https://doi.org/10.1007/s00586-020-06398-4
Murphy RF, Mooney JF (2017) The crankshaft phenomenon. J Am Acad Orthop Surg 25:e185–e193. https://doi.org/10.5435/JAAOS-D-16-00584
Chang D-G, Suk S-I, Kim J-H et al (2019) Long-term outcome of selective thoracic fusion using rod derotation and direct vertebral rotation in the treatment of thoracic adolescent idiopathic scoliosis more than 10-year follow-up data. Clin Spine Surg 33(22):E50–E57
Fletcher ND, McClung A, Rathjen KE et al (2012) Serial casting as a delay tactic in the treatment of moderate-to-severe early-onset scoliosis. J Pediatr Orthop 32:664–671. https://doi.org/10.1097/BPO.0b013e31824bdb55
Lenke LG, Betz RR, Harms J, Bridwell KH, Clements DH, Lowe TG, Blanke K (2001) Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Jt Surg Am 83:1169–1181. https://doi.org/10.2106/00004623-200108000-00006
D’Amato CR, Griggs S, McCoy B (2001) Nighttime bracing with the Providence brace in adolescent girls with idiopathic scoliosis. Spine (Phila Pa 1976) 26:2006–2012
Cheh G, Bridwell KH, Lenke LG et al (2007) Adjacent segment disease following lumbar/thoracolumbar fusion with pedicle screw instrumentation. Spine (Phila Pa 1976) 32:2253–2257. https://doi.org/10.1097/BRS.0b013e31814b2d8e
Roberto RF, Lonstein JE, Winter RB, Denis F (1997) Curve progression in Risser stage 0 or 1 patients after posterior spinal fusion for idiopathic scoliosis. J Pediatr Orthop 17:718–725. https://doi.org/10.1097/01241398-199711000-00005
Sanders JO, Herring JA, Browne RH (1995) Posterior arthrodesis and instrumentation in the immature (Risser-grade-0) spine in idiopathic scoliosis. J Bone Joint Surg 77:39–45. https://doi.org/10.2106/00004623-199501000-00006
Cherian D, Samdani AF, Schüpper AJ et al (2023) Early outcomes in hybrid fixation for idiopathic scoliosis: posterior fusion combined with anterior vertebral body tethering. Patient series. J Neurosurg: Case Lessons 6. https://doi.org/10.3171/CASE23331
Samdani AF, Ames RJ, Kimball JS et al (2014) Anterior vertebral body tethering for idiopathic scoliosis: Two-year results. Spine (Phila Pa 1976) 39:1688–1693. https://doi.org/10.1097/BRS.0000000000000472
Baker CE, Milbrandt TA, Larson AN (2021) Anterior vertebral body tethering for adolescent idiopathic scoliosis: early results and future directions. Orthop Clin North Am 52:137–147
Rushton PRP, Nasto L, Parent S et al (2021) Anterior vertebral body tethering for treatment of idiopathic scoliosis in the skeletally immature: results of 112 cases. Spine (Phila Pa 1976) 46:1461–1467. https://doi.org/10.1097/BRS.0000000000004061
Raitio A, Syvänen J, Helenius I (2022) Vertebral body tethering: indications, surgical technique, and a systematic review of published results. J Clin Med 11:2576
Roser MJ, Askin GN, Labrom RD et al (2023) Vertebral body tethering for idiopathic scoliosis: a systematic review and meta-analysis. Spine Deform 11(6):1297–1307
Seleviciene V, Cesnaviciute A, Strukcinskiene B et al (2022) Physiotherapeutic scoliosis-specific exercise methodologies used for conservative treatment of adolescent idiopathic scoliosis, and their effectiveness: an extended literature review of current research and practice. Int J Environ Res Public Health 19:9240. https://doi.org/10.3390/ijerph19159240
Qin X, Sun W, Xu L et al (2016) Selecting the last “substantially” touching vertebra as lowest instrumented vertebra in Lenke Type 1A curve. Spine (Phila Pa 1976) 41:E742–E750. https://doi.org/10.1097/BRS.0000000000001374
Menger RP, Park P, Konigsberg M et al (2020) Choice of Lowest Instrumented Vertebra (LIV) in Adolescent Idiopathic Scoliosis (AIS); Discordance in stable sagittal vertebra and coronal last touched vertebra. Neurosurg 87(Supplement_1). https://doi.org/10.1093/neuros/nyaa447_784
Beauchamp EC, Lenke LG, Cerpa M et al (2020) Selecting the “touched vertebra” as the lowest instrumented vertebra in patients with Lenke Type-1 and 2 curves radiographic results after a minimum 5-year follow-up. J Bone Jt Surg 102(22):1966–1973
Ding R, Liang J, Qiu G et al (2014) Evaluation of quality of life in adolescent idiopathic scoliosis with different distal fusion level. J Spinal Disord Tech 27:E155–E161. https://doi.org/10.1097/BSD.0000000000000073
Ghandehari H, Mahabadi MA, Mahdavi SM et al (2015) Evaluation of patient outcome and satisfaction after surgical treatment of adolescent idiopathic scoliosis using scoliosis research society-30. Arch Bone Jt Surg 3:109–113
Djurasovic M, Glassman S, Sucato D et al (2016) Improvement in SRS-22R pain scores after surgery for adolescent idiopathic scoliosis. Spine Journal 16:343–344
Fischer CR, Lenke LG, Bridwell KH et al (2018) Optimal lowest instrumented vertebra for thoracic adolescent idiopathic scoliosis. Spine Deform 6:250–256. https://doi.org/10.1016/j.jspd.2017.10.002
Funding
Open access funding provided by National Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics
The study was approved by the regional ethical committee and the local data protection agency.
Conflicts of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Disclosures
No disclosures to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ohrt-Nissen, S., Heegaard, M., Andersen, T. et al. Bracing in severe skeletally immature adolescent idiopathic scoliosis: does a holding strategy change the surgical plan?. Eur Spine J 33, 2457–2462 (2024). https://doi.org/10.1007/s00586-024-08246-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-024-08246-1