Abstract
Purpose
Sitting balance on an unstable surface requires coordinated out-of-phase lumbar spine and provides sufficient challenge to expose quality of spine control. We investigated whether the quality of spine coordination to maintain balance in acute low back pain (LBP) predicts recovery at 6 months.
Methods
Participants in an acute LBP episode (n = 94) underwent assessment of sitting balance on an unstable surface. Seat, hip and spine (lower lumbar, lumbar, upper lumbar, thoracic) angular motion and force plate data were recorded. Coordination between the seat and hip/spine segments to maintain balance was quantified in the frequency domain to evaluate coordination (coherence) and relative timing (phase angle: in-phase [segments move together]; out-of-phase [segments move opposite]). Center of pressure (CoP) and upper thorax motion assessed overall balance performance. Hip and spine coordination with the seat were compared between those who did not recover (increased/unchanged pain/disability), partially recovered (reduced pain/disability) or recovered (no pain and disability) at 6 months.
Results
In both planes, coherence between the seat and lower lumbar spine was lower (and in-phase—unhelpful for balance) at baseline in those who did not recover than those who recovered. Coherence between the seat and hip was higher in partially recovered in both planes, suggesting compensation by the hip. LBP groups had equal overall balance performance (CoP, upper thorax motion), but non-recovery groups used a less optimal strategy that might have consequences for long-term spine health.
Conclusion
These longitudinal data revealed that individuals with compromised contribution of the lumbar spine to the balance during unstable sitting during acute LBP are less likely to recover.
Similar content being viewed by others
Introduction
Low back pain (LBP) is a complex multi-factorial condition [1] and the leading cause of years lived with disability globally [2,3,4], with enormous economic impact [5, 6]. Although many individuals with new episodes of LBP recover quickly [1], recurrence is common [1, 7,8,9,10], and in some cases LBP becomes persistent and disabling [9, 11, 12]. Despite considerable efforts, LBP outcomes are not improving, and the global burden of LBP is projected to increase even further in coming decades [1, 4]. A better understanding of risk factors that underlie the transition from acute to chronic LBP would provide additional insights into LBP prevention/management.
Suboptimal loading on spine tissues because of poor quality trunk postural control has been suggested as a risk factor for the recurrence/persistence of LBP [13,14,15], but convincing evidence has not been forthcoming. Research is plagued by four major issues. First, most work has been cross-sectional [16,17,18,19,20,21,22] and unable to inform a potential role in outcome. Second, few studies [20, 21] have investigated trunk postural control early in the acute phase of LBP. Third, methods have been neither sufficiently challenging nor sufficiently precise to enable isolated consideration of the trunk (e.g., whole-body postural control in standing [23,24,25,26,27,28,29]) without compensation or contribution from other body segments. Fourth, even studies with unstable sitting paradigm have focused on overall balance performance (e.g., center of pressure [CoP] motion) [16, 17, 19,20,21,22] or straightforward kinematic measures of motion (e.g., range of motion) [16, 30,31,32] and lacked sophistication to reveal how spine motion is coordinated to overcome postural challenges. This study was designed to address these issues.
Trunk postural control involves maintenance of postural equilibrium (center of mass [CoM] over base of support) and upright orientation [33,34,35]. Both require precise balance between movement and stiffness to resist and overcome perturbations. Imprecise control could result in excessive spinal tissue load due to loss of balance or exaggerated muscle activity/responses [36]. There is considerable evidence of such changes once LBP has become chronic, with examples including delayed postural recovery in individuals who use less spine movement to maintain balance [37], increased spine stiffness [32, 38, 39] and delayed muscle response [19, 40].
Evaluation of postural control in unstable sitting (Fig. 1) enables interrogation of the trunk’s contribution to balance with limited contribution from the limbs [19, 41, 42], apart from a contribution by the hip. To maintain balance, the CoM is maintained close to the CoP via movements at the base/seat [43] induced by coordinated hip/spine movements [42, 44]. The lumbar spine has a primary role in maintaining balance in unstable sitting and is tightly coupled with seat movement (coherent movement) [42] (Fig. 2), but in the opposite direction (out-of-phase) to oppose any perturbations or to maintain the CoM close to the CoP [42, 45]. Recent work shows that some, but not all, individuals with acute LBP have less coherent motion between the lumbar spine and seat, and this motion is more in-phase, and thus unhelpful to oppose perturbations [46]. Movement of the spine with the seat suggests increased trunk stiffness because of exaggerated muscle co-contraction. Although this might represent a protective strategy to prevent movement of the painful region with short-term benefits [14, 30, 38, 47], the increased tissue load from muscle co-contraction and greater potential for balance loss might predispose individuals to further problems.
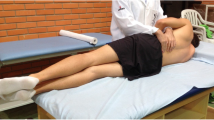
Experimental setup of the unstable sitting paradigm. Clusters of reflective markers were positioned over the spinous processes of L5/S1, L2/L3, T12/L1, T1, the lateral aspect of the left thigh, and attached to the back of the seat. The force plate was placed under the hemisphere. These clusters were used to define the following segments: hip (relative orientation between thigh and L5/S1 clusters), lower lumbar spine (relative orientation between L5/S1 and L2/L3 clusters), lumbar spine (relative orientation between L5/S1 and T12/L1 clusters), upper lumbar spine (relative orientation between L2/L3 and T12/L1 clusters) and thoracic spine (relative orientation between T12/L1 and T1 clusters)
Simplified depiction of coherent spine control in unstable sitting. Trunk postural control in anterior–posterior and medio-lateral directions is shown to illustrate the coherent lumbar out-of-phase strategy to maintain equilibrium during unstable sitting. In the sagittal plane, to restore equilibrium when the seat is tilted forward, the lumbar spine is extended and coherently moved out-of-phase (in the opposite direction to the seat), and the center of mass (CoM) of upper body in relation to the center of pressure (CoP) is returned to the equilibrium point. In the frontal plane, to restore equilibrium when the seat is tilted to the right, the lumbar spine is laterally flexed to the left and coherently moved out-of-phase (in the opposite direction to the seat), and similar to the sagittal plane, the CoM of upper body in relation to the CoP is returned to the equilibrium point
This study aimed to examine the hypothesis that individuals with a poorer quality coordination of the hip and spine during the early acute phase of LBP are more likely to develop persistent long-term pain and disability.
Materials and methods
Participants
Participants were from an established LBP cohort [48] and were included only if they were within 2 weeks of experiencing an acute episode of non-specific LBP. Participants had pain located between thoracolumbar junction and gluteal crease and lasted for at least 24 h. At the time of testing, they presented with pain and/or disability. Participants were excluded if they were < 18 or > 50 years old, had a serious spinal pathology, or major pain/injury in any region in the last year [48]. Cross-sectional seated balance data have been published from this cohort [42].
Power calculation
The participants included in this study were from an established LBP cohort with a sample size based on a more complex statistical analysis that requires a large sample size [48]. The analysis for the present study was pre-planned rather than exploratory. We undertook a power analysis based on the mean (1.09) and SD (0.45) of the coherence data at baseline for individuals with acute LBP [46]. For a power of 0.80 and significance of 5%, we would require 66 participants to detect a 20% difference between groups with different outcomes at 6 months.
Questionnaires
The Numeric Pain Rating Scale (NPRS) and Roland Morris Disability Questionnaire (RMDQ) were used to assess LBP intensity and LBP-related disability, respectively, at baseline and each subsequent fortnight for 6 months (Table 1). Both measures were assessed as the average score during the last week. Psychological questionnaires (Table 1) including the Pain Catastrophizing Scale (PCS) and Fear-Avoidance Beliefs Questionnaire (FABQ) were assessed at baseline only.
Experimental setup
Movement of the hip and spine while controlling postural balance was evaluated using an unstable sitting paradigm [19, 41, 42]. This paradigm (Fig. 1) has: (1) an aluminum hemisphere (radius: 250 mm; height of seat: 195 mm) attached underneath the seat and enables rotational movement in three degrees of freedom [16, 42]; (2) an adjustable footplate attached to the seat (90° knee flexion) for limiting lower limbs’ contribution to balance; and (3) front and side rails placed close to the seat. To estimate orientation of the spine, pelvis, thigh and seat, non-collinear clusters of four reflective markers were attached to the skin over L5/S1, L2/L3, T12/L1 and T1 spinous processes, and lateral left thigh and the back of seat (Fig. 1). Vicon (8-camera, at 100 samples/s; T40, Vicon Industries, Inc., New York, USA) or OptiTrack (10-camera, at 120 samples/s; Flex 13, NaturalPoint, Inc., Corvallis, USA) system was used to track 3D positions of the markers.
Experimental procedure
Trunk postural control was evaluated at baseline in two visual conditions: eyes open and eyes closed. The latter was included to render the task more challenging by demanding the use of sensory inputs other than vision. Participants were instructed to “sit upright as quietly as possible.” Six 30-s trials were recorded, three for each visual condition, each trial separated by ~ 30-s rest, and the order of each condition was randomized. Safety rail touch/hold was recorded for further analysis.
Data analysis
MATLAB (R2019a, Mathworks Inc., Natick, MA, USA) was used to analyze data. The x, y, and z coordinates of markers were filtered with a Butterworth filter (4th-order, bi-directional, low-pass) at 2.5 Hz [16]. OptiTrack data were resampled at 100 samples/s using spline interpolation.
The relative orientation between clusters was determined by multiplying the cranial cluster quaternion by the conjugate of the caudal cluster quaternion at each sample to extract the following segments: hip (relative orientation between thigh and L5/S1 clusters), lower lumbar spine (relative orientation between L5/S1 and L2/L3 clusters), lumbar spine (relative orientation between L5/S1 and T12/L1 clusters), upper lumbar spine (relative orientation between L2/L3 and T12/L1 clusters) and thoracic spine (relative orientation between T12/L1 and T1 clusters). Further details regarding the methods have been described previously [42]. Cross-spectral (frequency domain) analysis was performed to quantify the relation between changes in global seat orientation and changes in angle of the hip and spine regions in sagittal and frontal planes. This analysis enables the extraction of two outcome measures: phase angle and coherence. Phase angle describes the relative change of two signals to each other at different frequencies and has two distinct relations: in-phase and out-of-phase [49]. In-phase relation indicates that the phase difference between two signals is closer to 0º (both signals tend change together; same direction), and out-of-phase relation indicates that the phase difference between two signals is closer to 180º (one signal has approximately half a cycle delay relative to the other; opposite direction). Coherence describes the correlation between two signals and enables the determination of whether phase angle has a consistent (coherence = 1) or inconsistent (coherence = 0) relation [50]. Coherence significance threshold was set at 0.4856 [50]. Data from the three 30-s trials for each visual condition were combined; then, the means of both phase angle (using circular mean) and coherence were determined in the 0–0.5 Hz frequency band.
Motion at the base (CoP) and upper thorax were calculated to assess overall balance performance. Force plate data sampled at 2000 samples/s were filtered using a Butterworth filter (4th-order, bi-directional, low-pass) at 20 Hz cutoff frequency and then decimated at 100 samples/s. The mean CoP position was subtracted, and the root-mean-square amplitude (RMSdisplacement) and velocity (RMSvelocity) of the CoP in sagittal and frontal planes were calculated. Normalized upper thorax motion was calculated as sway path of the mean T1 cluster position normalized to spine length (the distance between T1 cluster and seat surface).
Statistical analysis
Statistical analyses were performed using StataIC 16 (Release 16, StataCorp LLC, College Station, TX). A P-value < 0.05 was considered significant.
Participants were categorized based on their 6-month pain and disability status into three groups: (1) Unrecovered—increased or unchanged pain/disability from baseline at 6 months (n = 15), (2) Partially recovered—decreased but not fully resolved pain and/or disability from baseline (n = 60), and (3) Recovered—no pain/disability at 6 months (n = 19). Six-month pain/disability status was calculated by averaging the final three (weeks 20, 22 and 24) NPRS and RMDQ scores.
Questionnaire data were compared between unrecovered, partially recovered and recovered groups using Fisher’s exact tests (for categorical variables) or one-way ANOVAs (for continuous variables) with Bonferroni post hoc analyses.
Generalized Estimating Equations (GEEs) using exchangeable methods (with robust estimation of standard errors) were performed to analyze the outcomes of coordination between the movement of each segment in relation to seat movement (phase angle, coherence) and the outcomes of overall balance performance (RMSdisplacement, RMSvelocity, normalized upper thorax motion). The analysis was performed separately for each Segment (only for phase angle and coherence; hip, lower lumbar, lumbar, upper lumbar, thoracic) and Plane (sagittal, frontal) to compare between Group (recovered, partially recovered, unrecovered) and Visual Condition (eyes open, eyes closed) with their interactions entered as fixed factors. Body mass index (BMI) [18, 41, 42, 51] and the frequency at which participants touched the safety rail [42] were included as confounding variables. BMI was mean-centered, phase angle values were transformed to absolute values, and coherence values were Fisher-transformed before analyses to achieve normal distribution. Non-significant interactions were omitted from models. In case of significant interactions involving Group, Bonferroni post hoc analyses were performed. Similar GEEs were performed to include additional covariates to determine the relationship between coordination outcomes and pain/disability change scores (degree of recovery from baseline to 6 months with respect to pain/disability).
To further investigate the relationship between measures (pain/disability change score) and coordination outcomes (phase angle, coherence), independent of our allocation to recovery groups (recovered, partially recovered, unrecovered), we conducted pairwise correlation analyses separately for each Segment and Plane.
Results
Participant characteristics
Data from 94 participants who completed follow-up pain/disability testing at 6 months were analyzed (Table 2). At baseline, LBP groups differed in BMI (post hoc: unrecovered < recovered [P = 0.029]) and PCS scores (post hoc: unrecovered [P < 0.001] and partially recovered [P = 0.011] > recovered). According to our recovery definition, LBP groups differed in terms of absolute pain/disability scores and pain/disability change scores at 6 months (post hoc: unrecovered [all, P < 0.001] and partially recovered [all, P < 0.05] > recovered, unrecovered [all, P < 0.001] > partially recovered).
Sagittal hip/spine coordination
Coherence between the seat and lower lumbar spine segment was lower in partially recovered (P = 0.015) and unrecovered (P = 0.009) groups than in the recovered group, regardless of visual condition (Table 3, Fig. 3; Supplementary Table 1 [post hoc results]), suggesting less consistent contribution of the lower lumbar spine to balance control. In contrast, coherence between the seat and hip segment was higher in the partially recovered than the recovered group during eyes open (P = 0.046; Table 3, Fig. 3; Supplementary Table 1), suggesting compensation by the hip for this group. In conjunction with lower coherence of the lower lumbar spine, participants in the unrecovered group moved the lumbar spine segment more toward the same direction as the seat (moving further to in-phase; e.g., lumbar flexion during forward seat tilt; unhelpful for balance recovery), in contrast to out-of-phase movement (helpful for balance recovery) by the recovered group, regardless of visual condition (P = 0.009, Table 3, Fig. 3; Supplementary Table 1). There was a small, but significant, positive relationship between lumbar coherence and disability change score (P = 0.028; Supplementary Table 2 and Fig. 1 [relationships results using GEE models]).
Sagittal plane marginal mean differences in phase angle and coherence with seat movements between the three LBP groups. The differences in phase angle and coherence between recovered (green), partially recovered (orange) and unrecovered (red) groups for each segment (hip, lower [L.] lumbar, lumbar, upper [U.] lumbar, thoracic) and visual condition (eyes closed [EC], eyes open [EO]) are shown in both planes (sagittal plane, frontal plane). Error bars represent 95% confidence intervals. In-phase (0°) relation indicates that the segment moves in the same direction as the seat, and out-of-phase (180°) relation indicates that the segment moves in the opposite direction to the seat. The significance threshold for coherence (0.4856) is shown in the horizontal dashed gray line. Higher coherence values indicate more consistent hip/spine phase relation with seat movements. Brackets indicate P < 0.05 between groups
Frontal hip/spine coordination
Similar to the sagittal plane, coherence between the seat and lumbar spine segment was lower in the unrecovered than the recovered group, regardless of visual condition (P = 0.006; Table 3, Fig. 4; Supplementary Table 1). Coherence between the seat and hip segment was higher in the partially recovered than the recovered group, regardless of visual condition (P = 0.023; Table 3, Fig. 4; Supplementary Table 1). Participants in the unrecovered group used less out-of-phase movement of the lumbar spine segment with the seat (e.g., lumbar left lateral flexion during right seat tilt) than participants in the recovered group, regardless of visual condition (P = 0.024, Table 3, Fig. 4; Supplementary Table 1). There was a significant negative relationship between lumbar coherence and pain change scores (P = 0.002; Supplementary Table 2 and Fig. 1), suggesting that higher coherence is associated with greater improvement in pain.
Frontal plane marginal mean differences in phase angle and coherence with seat movements between the three LBP groups. The differences in phase angle and coherence between recovered (green), partially recovered (orange) and unrecovered (red) groups for each segment (hip, lower [L.] lumbar, lumbar, upper [U.] lumbar, thoracic) and visual condition (eyes closed [EC], eyes open [EO]) are shown in both planes (sagittal plane, frontal plane). Error bars represent 95% confidence intervals. In-phase (0°) relation indicates that the segment moves in the same direction as the seat, and out-of-phase (180°) relation indicates that the segment moves in the opposite direction to the seat. The significance threshold for coherence (0.4856) is shown in the horizontal dashed gray line. Higher coherence values indicate more consistent hip/spine phase relation with seat movements. Brackets indicate P < 0.05 between groups
Pairwise correlations between hip/spine coordination and pain/disability change scores
Pain (all, P < 0.001) and disability (all, P < 0.05) change scores were negatively correlated with coherence between the lower lumbar spine and seat, in both planes (Supplementary Table 3 and Figs. 2 and 3 [Pairwise correlations results]), i.e., greater improvement in clinical outcomes in those with higher coherence. Pain change scores were also negatively correlated with phase angle (all, P < 0.05) and coherence (all, P < 0.05) of the lumbar spine with the seat in both planes (Supplementary Table 3 and Figs. 2 and 3), i.e., greater improvement in clinical outcomes in those with coherent and out-of-phase movement. Further, pain change score was negatively correlated with phase angle of the upper lumbar spine (P = 0.042; Supplementary Table 3 and Fig. 2) and disability change score was negatively correlated with the coherence of thoracic spine (P = 0.005; Supplementary Table 3 and Fig. 3) in the frontal plane.
Overall balance performance
No significant between-group differences were found for RMSdisplacement, RMSvelocity and normalized upper thorax motion (Table 4, Fig. 5), which implies that all LBP groups had similar success in overall balance performance.
Mean differences in overall balance performance between the three LBP groups. The differences in RMSdisplacement, RMSvelocity and normalized upper thorax motion between recovered (green), partially recovered (orange) and unrecovered (red) groups for each visual condition (eyes closed [EC], eyes open [EO]) are shown in both planes (sagittal plane, frontal plane). Error bars represent 95% confidence intervals
Discussion
This study is the first to show that a less optimal use of lumbar spine motion to maintain balance in the acute phase of LBP predicts persistence of symptoms in the longer term. Our experimental balance task provided sufficient challenge to expose differences in the strategy for spine control between outcome groups, and our innovative analysis methods revealed inter-related observations that corroborate this novel interpretation. Detailed analyses in the frequency domain using GEE models revealed that, compared to participants who had recovered at 6 months, those who did not fully recover used: (i) less consistent motion of the lumbar spine (lower coherence with seat movement) to counteract balance disturbance from seat movement, (ii) lumbar spine motion that was more in-phase with the seat and thus unhelpful for balance recovery and (iii) more consistent motion of the hip, perhaps as a compensation for poor coordination of the lumbar spine. Further corroborating the outcomes, pairwise correlations showed that lower coherence of the lumbar spine and movement more toward in-phase is correlated with lesser improvement (pain/disability change scores) at 6 months. These findings have implications for the interpretation of underlying mechanisms for the recurrence/persistence of LBP with potential relevance for the design of targeted approaches to LBP treatment.
Interpretation of differences between sub-groups in spine coordination strategy
Our findings suggest that the overall balance performance measured from conventional time domain analyses such as CoP motion was similar between LBP groups, but how this was achieved (coordination strategy) differed between the recovery groups (recovered, partially recovered, unrecovered). Recent individual participant data meta-analysis has shown greater RMS CoP displacements in individuals with acute and persistent LBP compared to pain-free individuals (Alshehri et al., 2023 unpublished data), but no differences within the LBP population have been shown. A benefit of detailed frequency domain analysis of the spine and seat movement is the capacity to understand how spine motion is coordinated to achieve balance. Previous work has shown that although pain-free individuals use coherent out-of-phase motion of the lumbar spine in both planes to maintain balance [42], coherent motion is generally less in individuals with acute LBP [46], but with some within-group variation of coherence. The present analysis extends this observation to show that this variation relates to recovery. The less consistent more in-phase motion of the lumbar spine in the sagittal plane is unhelpful for balance recovery as it moves with the seat. Failure to move opposite to perturbations might be a consequence of a strategy to limit spine movement, thus prioritizing spine protection over balance control. This strategy would increase spine stiffness due to increased muscle co-activation [38, 52, 53] and/or enhanced control of lower trunk admittance (i.e., less displacement per unit of applied force as a result of higher position, velocity and acceleration feedback gains) from higher reflex gains [39]. Alternatively, if spine proprioception is impaired/reduced, as is common in LBP [54,55,56,57], it would reduce the ability of spine muscles to adequately respond to balance disturbances [19, 40], and thus spine movement might be limited to avoid error. Here successful balance was enabled by greater coordination (higher coherence) between the hip and seat. Similar compensation strategies for reducing spine movement through use of the lower limbs have been shown in other balance paradigms such as multidirectional support surface translations in standing [58] and when healthy individuals are exposed to experimental pain [59].
In the frontal plane, results are generally similar, or even more robust. In this plane, the hip can only make a trivial contribution and balance is more dependent on spine movement. Previous work has shown that in this plane, most lumbar coherence values exceed the significance threshold, which implies that the lumbar spine is more important for frontal plane balance control during unstable sitting than other segments [42]. Despite this importance, unrecovered participants adopted a less consistent and less out-of-phase lumbar strategy which again implies limitation of spine motion either to protect or limit error.
Potential relevance of differences in spine coordination strategy for LBP recovery
Current data cannot confirm whether the strategy adopted in the acute period is causally related to the persistence of LBP or related to some other factor that explains both the different strategy and poor outcome. However, there is reason to speculate that the less optimal strategy might cause persistence. One possible explanation is that if the lesser spine motion is related to increased muscle co-contraction to either protect the spine from further injury or to limit error secondary to poor proprioception, this would increase spine loading as has been speculated [14] and shown in other tasks in cross-sectional studies [47, 60]. Even though it is challenging to entirely ascertain the existence of ongoing noxious stimulation or microtrauma on spine tissues, the plausibility of a causal relationship between pain persistence in LBP and spine tissue loading cannot be refuted [61]. Connective tissues are richly innervated with nociceptive neurons [62]. Nociceptive inputs are driven, primarily, by the activation of specialized peripheral sensory neurons (nociceptors) [63]. In the spine, ongoing nociceptive inputs in response to increased spine tissue loading may initiate changes in the central nervous system that drive pain persistence [64]. In this case, the restricted (restrained) spine motion might maintain the activation of nociceptive inputs that might persist beyond an acute injury phase. Other longitudinal work has related cumulative low back loads to the occurrence of LBP [65], although in that work loading was related to the position (e.g., working in a trunk-flexed posture) and the frequency of task performance (e.g., number of lifts during work). Another possibility is that the suboptimal balance strategy adopted by the unrecovered group renders balance control less robust with greater potential for balance loss, excessive spine motion, excessive spine loading during recovery and injury. These alternatives require further exploration.
The present findings provide additional interpretation of the few longitudinal studies that have investigated the relationship between trunk postural control and the development or recurrence/persistence of LBP. One study showed greater risk of developing future LBP in athletes with delayed deactivation of trunk muscle activity after quick force release in a semi-seated position [66]. This evidence of augmented activity could relate to the restrained uncoherent, more in-phase spine motion after perturbations in our study. Other data showed that symptom-free adults with greater CoP displacements during vibration of the soleus muscles (for proprioception disturbance) in standing (similar to individuals with LBP), had a higher risk of developing or having a recurrent course of LBP [67]. Greater response to soleus vibration has been attributed to a reliance on an ankle rather than lumbar spine strategy for balance control [68], which concurs with less lumbar contribution in the present study. Together these data support the notion that compromised dynamic control of the lumbar spine is a risk factor for the persistence of LBP.
Clinical implications
These findings have several implications. First, assessment of the balance strategy in acute LBP might provide a method to predict clinical outcomes. Although plausible, it is critical to acknowledge that LBP is a multifactorial condition and many other biological, psychological and social features will contribute to the prediction of outcome and the trajectory of pain. Previous analyses of this LBP cohort have identified relationships between baseline biological (e.g., inflammatory profiles [69] and central sensitization signs [70]), psychological (e.g., depressive symptoms) and social (e.g., impending compensation [71]) features. A multifactorial analysis is likely to provide the most accurate prediction of outcome.
Second, the trunk’s contribution to balance control is potentially modifiable by training. Other features of trunk motor control are modifiable with exercise [72, 73]. Although it is possible that improving the trunk’s contribution to balance control might promote clinical improvement, consideration of other features due to the multidimensional nature of LBP is likely to be essential for greater improvement in clinical outcomes. Of course, the potential efficacy of any intervention depends on the confirmation of a causal link between the balance strategy and clinical outcomes.
Methodological considerations
Several methodological issues must be mentioned. First, recovery outcome was determined at 6 months and analyses over a longer term (e.g., 12 months) are worthy of consideration. Second, although based on previous analyses [48], definitions of recovery are somewhat arbitrary. Different outcomes might be identified if different thresholds were specified. Third, LBP sub-group (based on recovery groups) sizes varied and some were small. Greater confidence would be gained from replication of our results in an independent cohort. Fourth, phase angle data have a robust meaningful interpretation when the coherence is above the significance threshold and a less meaningful interpretation when the coherence is low or below the significance threshold. This limitation requires consideration for interpretation of the findings of the lower lumbar and lumbar spine in the sagittal plane where coherence was not high. Fifth, although a relatively large number of variables were included in the analysis, a small (but quite consistent) set of variables showed an effect of group. Sixth, we did not measure whether spinal alignment (e.g., the radiological measure of sagittal balance) influenced the trunk postural control measures. Although we cannot exclude that this might influence our outcomes, this would not change the major findings of this study.
Conclusions
Using innovative methods and analyses, this study provides novel data indicating that the trunk’s contribution to balance control in acute LBP is related to outcome at 6 months. These findings have potential relevance for treatment design and prediction of outcome.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, Hoy D, Karppinen J, Pransky G, Sieper J (2018) What low back pain is and why we need to pay attention. Lancet 391:2356–2367. https://doi.org/10.1016/S0140-6736(18)30480-X
Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abdulkader RS, Abdulle AM, Abebo TA, Abera SF (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390:1211–1259. https://doi.org/10.1016/S0140-6736(17)32154-2
James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1789–1858. https://doi.org/10.1016/S0140-6736(18)32279-7
Ferreira ML, de Luca K, Haile LM, Steinmetz JD, Culbreth GT, Cross M, Kopec JA, Ferreira PH, Blyth FM, Buchbinder R (2023) Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol 5:e316–e329. https://doi.org/10.1016/S2665-9913(23)00098-X
Fatoye F, Gebrye T, Ryan CG, Useh U, Mbada C (2023) Global and regional estimates of clinical and economic burden of low back pain in high-income countries: a systematic review and meta-analysis. Front Public Health 11:1098100. https://doi.org/10.3389/fpubh.2023.1098100
Fatoye F, Gebrye T, Mbada CE, Useh U (2023) Clinical and economic burden of low back pain in low-and middle-income countries: a systematic review. BMJ Open 13:e064119. https://doi.org/10.1136/bmjopen-2022-064119
Hestbaek L, Leboeuf-Yde C, Manniche C (2003) Low back pain: what is the long-term course? A review of studies of general patient populations. Eur Spine J 12:149–165. https://doi.org/10.1007/s00586-002-0508-5
Leboeuf-Yde C, Grønstvedt A, Borge JA, Lothe J, Magnesen E, Nilsson Ø, Røsok G, Stig L-C, Larsen K (2005) The Nordic back pain subpopulation program: a 1-year prospective multicenter study of outcomes of persistent low-back pain in chiropractic patients. J Manipulative Physiol Ther 28:90–96. https://doi.org/10.1016/j.jmpt.2005.01.010
Hayden J, Dunn K, Van der Windt D, Shaw W (2010) What is the prognosis of back pain? Best Pract Res Clin Rheumatol 24:167–179. https://doi.org/10.1016/j.berh.2009.12.005
Pengel LH, Herbert RD, Maher CG, Refshauge KM (2003) Acute low back pain: systematic review of its prognosis. BMJ 327:323. https://doi.org/10.1136/bmj.327.7410.323
Klenerman L, Slade P, Stanley I, Pennie B, Reilly J, Atchison L, Troup J, Rose M (1995) The prediction of chronicity in patients with an acute attack of low back pain in a general practice setting. Spine 20:478–484. https://doi.org/10.1097/00007632-199502001-00012
Patrick N, Emanski E, Knaub MA (2014) Acute and chronic low back pain. Med Clin North Am 98:777–789. https://doi.org/10.1016/j.mcna.2014.03.005
Ebenbichler GR, Oddsson LI, Kollmitzer J, Erim Z (2001) Sensory-motor control of the lower back: implications for rehabilitation. Med Sci Sports Exerc 33:1889–1898. https://doi.org/10.1097/00005768-200111000-00014
Hodges PW, Tucker K (2011) Moving differently in pain: a new theory to explain the adaptation to pain. Pain 152:S90–S98. https://doi.org/10.1016/j.pain.2010.10.020
Hodges PW, Danneels L (2019) Changes in structure and function of the back muscles in low back pain: different time points, observations, and mechanisms. J Orthop Sports Phys Ther 49:464–476. https://doi.org/10.2519/jospt.2019.8827
Willigenburg NW, Kingma I, van Dieën JH (2013) Center of pressure trajectories, trunk kinematics and trunk muscle activation during unstable sitting in low back pain patients. Gait Posture 38:625–630. https://doi.org/10.1016/j.gaitpost.2013.02.010
Cyr KM, Wilson SE, Mehyar F, Sharma NK (2019) Trunk control response to unstable seated posture during various feedback conditions in people with chronic low back pain. J Allied Health 48:54–60. https://www.jstor.org/stable/48723136
Larivière C, Gagnon DH, Mecheri H (2015) Trunk postural control in unstable sitting: effect of sex and low back pain status. Clin Biomech 30:933–939. https://doi.org/10.1016/j.clinbiomech.2015.07.006
Radebold A, Cholewicki J, Polzhofer GK, Greene HS (2001) Impaired postural control of the lumbar spine is associated with delayed muscle response times in patients with chronic idiopathic low back pain. Spine 26:724–730. https://doi.org/10.1097/00007632-200104010-00004
van den Hoorn W, Meroni R, Klyne DM, Alshehri MA, Hodges PW (2022) Balance control in unstable sitting in individuals with an acute episode of low back pain. Gait Posture 95:15–21. https://doi.org/10.1016/j.gaitpost.2022.03.014
Sung W, Abraham M, Plastaras C, Silfies SP (2015) Trunk motor control deficits in acute and subacute low back pain are not associated with pain or fear of movement. Spine J 15:1772–1782. https://doi.org/10.1016/j.spinee.2015.04.010
Navalgund AR (2009) Evaluating the effect of a 10-week stabilization exercise program on the postural stability and the neuromuscular control of the spine in subjects with subacute recurrent low back pain. Dissertation, The Ohio State University
Prins MR, Griffioen M, Veeger TT, Kiers H, Meijer OG, van der Wurff P, Bruijn SM, van Dieën JH (2018) Evidence of splinting in low back pain? A systematic review of perturbation studies. Eur Spine J 27:40–59. https://doi.org/10.1007/s00586-017-5287-0
Mazaheri M, Coenen P, Parnianpour M, Kiers H, van Dieën JH (2013) Low back pain and postural sway during quiet standing with and without sensory manipulation: a systematic review. Gait Posture 37:12–22. https://doi.org/10.1016/j.gaitpost.2012.06.013
Berenshteyn Y, Gibson K, Hackett GC, Trem AB, Wilhelm M (2018) Is standing balance altered in individuals with chronic low back pain? A systematic review. Disabil Rehabil 41:1514–1523. https://doi.org/10.1080/09638288.2018.1433240
Ruhe A, Fejer R, Walker B (2011) Center of pressure excursion as a measure of balance performance in patients with non-specific low back pain compared to healthy controls: a systematic review of the literature. Eur Spine J 20:358–368. https://doi.org/10.1007/s00586-010-1543-2
Koch C, Hänsel F (2019) Non-specific low back pain and postural control during quiet standing—a systematic review. Front Psychol 10:586. https://doi.org/10.3389/fpsyg.2019.00586
Park J, Nguyen VQ, Ho RL, Coombes SA (2023) The effect of chronic low back pain on postural control during quiet standing: a meta-analysis. Sci Rep 13:7928. https://doi.org/10.1038/s41598-023-34692-w
Knox MF, Chipchase LS, Schabrun SM, Romero RJ, Marshall PW (2018) Anticipatory and compensatory postural adjustments in people with low back pain: a systematic review and meta-analysis. Spine J 18:1934–1949. https://doi.org/10.1016/j.spinee.2018.06.008
Freddolini M, Strike S, Lee RYW (2014) The role of trunk muscles in sitting balance control in people with low back pain. J Electromyogr Kinesiol 24:947–953. https://doi.org/10.1016/j.jelekin.2014.09.009
Freddolini M, Strike S, Lee R (2014) Dynamic stability of the trunk during unstable sitting in people with low back pain. Spine 39:785–790. https://doi.org/10.1097/BRS.0000000000000296
Van Daele U, Hagman F, Truijen S, Vorlat P, Van Gheluwe B, Vaes P (2009) Differences in balance strategies between nonspecific chronic low back pain patients and healthy control subjects during unstable sitting. Spine 34:1233–1238. https://doi.org/10.1097/BRS.0b013e31819ca3ee
Horak FB (1987) Clinical measurement of postural control in adults. Phys Ther 67:1881–1885. https://doi.org/10.1093/ptj/67.12.1881
Horak FB (2006) Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing 35:ii7-ii11. https://doi.org/10.1093/ageing/afl077
Massion J (1994) Postural control system. Curr Opin Neurobiol 4:877–887. https://doi.org/10.1016/0959-4388(94)90137-6
Van Dieën JH, Reeves NP, Kawchuk G, Van Dillen LR, Hodges PW (2019) Motor control changes in low back pain: divergence in presentations and mechanisms. J Orthop Sports Phys Ther 49:370–379. https://doi.org/10.2519/jospt.2019.7917
Mok NW, Brauer SG, Hodges PW (2011) Changes in lumbar movement in people with low back pain are related to compromised balance. Spine 36:45–52. https://doi.org/10.1097/BRS.0b013e3181dfce83
Radebold A, Cholewicki J, Panjabi MM, Patel TC (2000) Muscle response pattern to sudden trunk loading in healthy individuals and in patients with chronic low back pain. Spine 25:947–954. https://doi.org/10.1097/00007632-200004150-00009
Griffioen M, van Drunen P, Maaswinkel E, Perez R, Happee R, van Dieën J (2020) Identification of intrinsic and reflexive contributions to trunk stabilization in patients with low back pain: a case–control study. Eur Spine J 29:1900–1908. https://doi.org/10.1007/s00586-020-06385-9
Reeves NP, Cholewicki J, Narendra KS (2009) Effects of reflex delays on postural control during unstable seated balance. J Biomech 42:164–170. https://doi.org/10.1016/j.jbiomech.2008.10.016
Cholewicki J, Polzhofer G, Radebold A (2000) Postural control of trunk during unstable sitting. J Biomech 33:1733–1737. https://doi.org/10.1016/s0021-9290(00)00126-3
Alshehri MA, van den Hoorn W, Klyne D, Hodges PW (2021) Coordination of hip and spine to maintain equilibrium in unstable sitting revealed by spectral analysis. J Neurophysiol 125:1814–1824. https://doi.org/10.1152/jn.00555.2020
Roberts BW, Vette AH (2019) A kinematics recommendation for trunk stability and control assessments during unstable sitting. Med Eng Phys 73:73–76. https://doi.org/10.1016/j.medengphy.2019.08.004
Roberts BW, Gholibeigian F, Lewicke J, Vette AH (2020) Spatial and temporal relation of kinematics and muscle activity during unstable sitting. J Electromyogr Kinesiol 52:102418. https://doi.org/10.1016/j.jelekin.2020.102418
Acasio JC, Nussbaum MA, Hendershot BD (2021) Trunk-pelvic coordination during unstable sitting with varying task demand: a methodological study. J Biomech 118:110299. https://doi.org/10.1016/j.jbiomech.2021.110299
Alshehri MA, van den Hoorn W, Klyne DM, Hodges PW (2023) Coordination of hip and spine in individuals with acute low back pain during unstable sitting. Spine J. https://doi.org/10.1016/j.spinee.2023.12.001
Hodges P, van den Hoorn W, Dawson A, Cholewicki J (2009) Changes in the mechanical properties of the trunk in low back pain may be associated with recurrence. J Biomech 42:61–66. https://doi.org/10.1016/j.jbiomech.2008.10.001
Klyne DM, van den Hoorn W, Barbe MF, Cholewicki J, Hall LM, Khan A, Meroni R, Moseley GL, Nicholas M, O’Sullivan L (2020) Cohort profile: why do people keep hurting their back? BMC Res Notes 13:538. https://doi.org/10.1186/s13104-020-05356-z
Wang Z, Ko JH, Challis JH, Newell KM (2014) The degrees of freedom problem in human standing posture: collective and component dynamics. PLoS ONE 9:e85414. https://doi.org/10.1371/journal.pone.0085414
Halliday D, Rosenberg J, Amjad A, Breeze P, Conway B, Farmer S (1995) A framework for the analysis of mixed time series/point process data-theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol 64:237. https://doi.org/10.1016/s0079-6107(96)00009-0
Larivière C, Mecheri H, Shahvarpour A, Gagnon D, Shirazi-Adl A (2013) Criterion validity and between-day reliability of an inertial-sensor-based trunk postural stability test during unstable sitting. J Electromyogr Kinesiol 23:899–907. https://doi.org/10.1016/j.jelekin.2013.03.002
Reeves NP, Everding VQ, Cholewicki J, Morrisette DC (2006) The effects of trunk stiffness on postural control during unstable seated balance. Exp Brain Res 174:694–700. https://doi.org/10.1007/s00221-006-0516-5
van Dieën JH, Selen LP, Cholewicki J (2003) Trunk muscle activation in low-back pain patients, an analysis of the literature. J Electromyogr Kinesiol 13:333–351. https://doi.org/10.1016/s1050-6411(03)00041-5
Tong MH, Mousavi SJ, Kiers H, Ferreira P, Refshauge K, van Dieën J (2017) Is there a relationship between lumbar proprioception and low back pain? A systematic review with meta-analysis. Arch Phys Med Rehabil 98:120–136. https://doi.org/10.1016/j.apmr.2016.05.016
Lee AS, Cholewicki J, Reeves NP, Zazulak BT, Mysliwiec LW (2010) Comparison of trunk proprioception between patients with low back pain and healthy controls. Arch Phys Med Rehabil 91:1327–1331. https://doi.org/10.1016/j.apmr.2010.06.004
Parkhurst TM, Burnett CN (1994) Injury and proprioception in the lower back. J Orthop Sports Phys Ther 19:282–295. https://doi.org/10.2519/jospt.1994.19.5.282
Korakakis V, O’Sullivan K, Kotsifaki A, Sotiralis Y, Giakas G (2021) Lumbo-pelvic proprioception in sitting is impaired in subgroups of low back pain–but the clinical utility of the differences is unclear. A systematic review and meta-analysis. PLoS One 16:e0250673. https://doi.org/10.1371/journal.pone.0250673
Jones SL, Henry SM, Raasch CC, Hitt JR, Bunn JY (2012) Individuals with non-specific low back pain use a trunk stiffening strategy to maintain upright posture. J Electromyogr Kinesiol 22:13–20. https://doi.org/10.1016/j.jelekin.2011.10.006
Smith M, Coppieters MW, Hodges PW (2005) Effect of experimentally induced low back pain on postural sway with breathing. Exp Brain Res 166:109–117. https://doi.org/10.1007/s00221-005-2352-4
Ferguson SA, Marras WS, Burr DL, Davis KG, Gupta P (2004) Differences in motor recruitment and resulting kinematics between low back pain patients and asymptomatic participants during lifting exertions. Clin Biomech 19:992–999. https://doi.org/10.1016/j.clinbiomech.2004.08.007
Van Dieën JH, Reeves NP, Kawchuk G, Van Dillen LR, Hodges PW (2019) Analysis of motor control in patients with low back pain: a key to personalized care? J Orthop Sports Phys Ther 49:380–388. https://doi.org/10.2519/jospt.2019.7916
Langevin HM, Sherman KJ (2007) Pathophysiological model for chronic low back pain integrating connective tissue and nervous system mechanisms. Med Hypotheses 68:74–80. https://doi.org/10.1016/j.mehy.2006.06.033
Dubin AE, Patapoutian A (2010) Nociceptors: the sensors of the pain pathway. J Clin Invest 120:3760–3772. https://doi.org/10.1172/JCI42843
Panjabi MM (2006) A hypothesis of chronic back pain: ligament subfailure injuries lead to muscle control dysfunction. Eur Spine J 15:668–676. https://doi.org/10.1007/s00586-005-0925-3
Coenen P, Kingma I, Boot CR, Twisk JW, Bongers PM, van Dieën JH (2013) Cumulative low back load at work as a risk factor of low back pain: a prospective cohort study. J Occup Rehabil 23:11–18. https://doi.org/10.1007/s10926-012-9375-z
Cholewicki J, Silfies SP, Shah RA, Greene HS, Reeves NP, Alvi K, Goldberg B (2005) Delayed trunk muscle reflex responses increase the risk of low back injuries. Spine 30:2614–2620. https://doi.org/10.1097/01.brs.0000188273.27463.bc
Claeys K, Dankaerts W, Janssens L, Pijnenburg M, Goossens N, Brumagne S (2015) Young individuals with a more ankle-steered proprioceptive control strategy may develop mild non-specific low back pain. J Electromyogr Kinesiol 25:329–338. https://doi.org/10.1016/j.jelekin.2014.10.013
Brumagne S, Cordo P, Verschueren S (2004) Proprioceptive weighting changes in persons with low back pain and elderly persons during upright standing. Neurosci Lett 366:63–66. https://doi.org/10.1016/10.1016/j.neulet.2004.05.013
Klyne DM, Barbe MF, van den Hoorn W, Hodges PW (2018) ISSLS Prize in clinical science 2018: longitudinal analysis of inflammatory, psychological, and sleep-related factors following an acute low back pain episode—the good, the bad, and the ugly. Eur Spine J 27:763–777. https://doi.org/10.1007/s00586-018-5490-7
Klyne DM, Moseley GL, Sterling M, Barbe MF, Hodges PW (2019) Are signs of central sensitization in acute low back pain a precursor to poor outcome? J Pain 20:994–1009. https://doi.org/10.1016/j.jpain.2019.03.001
Klyne DM, Hall LM, Nicholas MK, Hodges PW (2022) Risk factors for low back pain outcome: Does it matter when they are measured? Eur J Pain 26:835–854. https://doi.org/10.1002/ejp.1911
Tsao H, Galea MP, Hodges PW (2010) Driving plasticity in the motor cortex in recurrent low back pain. Eur J Pain 14:832–839. https://doi.org/10.1016/j.ejpain.2010.01.001
Scholtes SA, Norton BJ, Lang CE, Van Dillen LR (2010) The effect of within-session instruction on lumbopelvic motion during a lower limb movement in people with and people without low back pain. Man Ther 15:496–501. https://doi.org/10.1016/j.math.2010.05.003
Acknowledgements
The authors acknowledge all researchers who helped with participant recruitment and data collection.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was funded by grants from the National Health and Medical Research Council (NHMRC) of Australia (631369; 1091302; 1194937).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Mansour Abdullah Alshehri], [David M. Klyne], [Wolbert van den Hoorn] and [Paul W. Hodges]. The first draft of the manuscript was written by [Mansour Abdullah Alshehri], and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Ethical approval was granted by the Institutional Human Research Ethics Committee (The University of Queensland), and all procedures were performed in accordance with the Declaration of Helsinki.
Consent to participate
All participants provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alshehri, M.A., van den Hoorn, W., Klyne, D.M. et al. Poor lumbar spine coordination in acute low back pain predicts persistent long-term pain and disability. Eur Spine J (2024). https://doi.org/10.1007/s00586-024-08205-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00586-024-08205-w