Abstract
Introduction
A recent study reported a 34% mid-term revision rate after M6-C™ cervical total disc replacement (CTDR) for wear-related osteolysis. Here, we aim to investigate the prevalence, risk factors, and radiographic characteristics of periprosthetic bony changes and implant failure of the M6-C™ artificial disc.
Methods
We retrospectively analysed radiographic (conventional X-ray, CT scan) and clinical outcomes (EQ-5D-5L, Neck Disability Index (NDI), and Visual Analog Scale (VAS) for neck and arm pain) data collected during routine follow-up of patients who underwent CTDR with the M6-C™ between 2011 and 2015.
Results
In total, 85 patients underwent CTDR with the M6-C™. Follow-up data were available for 43 patients (54% female, mean age 44 years) with 50 implants and a mean follow-up of 8.1 years (6.5–11 years). Implant failure with the presence of severe osteolysis was identified in 5 (12%) patients who were all male (p = 0.016) and implanted at the C5/6 level (p = 0.11). All failed implants required revision surgery. The overall prevalence of osteolysis was 44% (22/50 implants) and 34% (17/50 implants) for significant heterotopic ossification. Patients with high-grade osteolysis showed higher VAS arm pain (p = 0.05) and lower EQ-5D-VAS health VAS (p = 0.03).
Conclusion
We report a lower reoperation rate for failed M6-C™ implants than previously published, but confirmed that osteolysis and heterotopic ossification are common following CTDR with the M6-C™ and may be asymptomatic. Therefore, we strongly recommend ongoing clinical and radiographic monitoring after CTDR with the M6-C™, particularly for male patients implanted at the C5/6 level.
Similar content being viewed by others
Introduction
Anterior cervical decompression and fusion (ACDF) was first described in 1958. For several years, it utilised structural autologous iliac crest bone grafts, but polyether ether ketone (PEEK) and 3D-printed titanium now provide superior outcomes. While ACDF is effective in decompression, its drawbacks include increased disc degeneration in adjacent segments [1]. This is attributed to elevated intradiscal pressure and consequent compensatory mechanisms [2]. Such problems are lessened by cervical total disc replacement (CTDR) as it can preserve motion at the operated level [3].
Early cervical arthroplasty attempts, such as the Fernstom ball introduced in 1966, reported high rates of device-related complications [3]. However, recent advancements in designs have improved the safety of CTDR. Currently, nine artificial discs have been approved by the Food and Drug Administration (FDA) for single-level arthroplasty. These are categorised based on their degrees of freedom (DOF)—articulating versus non-articulating components [4, 5]. The M6-C™ artificial cervical disc (Orthofix, Lewisville, Texas) is a unique non-articulating unconstrained implant with a compliant core (mobile bumper design), allowing for all six DOF. The compressible artificial nucleus is made of polycarbonate urethane (PCU) with a woven fibre annulus made of Ultra-high-molecular-weight polyethylene (UHMWPE) designed to mimic native disc morphology and biomechanics (Fig. 1) [6]. The implant was approved by the FDA in 2019 for single-level CTDR and has been marketed outside the USA since 2006. Over 60,000 M6-C™ artificial discs have been implanted worldwide [7].
The M6-C™ artificial cervical disc implant comprises alloy (Ti6Al4V) outer and inner plates. Stability is ensured by two keels on each endplate. The implant features a sheath (polycarbonate urethane polymer—PCU) to prevent tissue ingrowth and debris migration. The fibre matrix represents the artificial annulus (ultra-high-molecular-weight polyethylene—UHMWPE), and the core is composed of the Artificial Nucleus (PCU) [14]
Despite the benefits of CTDR [8,9,10], complications including heterotopic ossifications (HO), subsidence, expulsion, dislocation, and osteolysis have been reported. Osteolysis is characterised by lytic end plate destruction and is linked to immunological responses to wear debris [11, 12]. Following a post-market review of the M6-C™ implant, the Australian Therapeutic Goods Administration (TGA) issued an Implant Hazard Alert, citing inadequate information in the instructions for use regarding the potential consequences of periprosthetic osteolysis [13]. Subsequently, the instructions were updated to include recommendations for clinical and radiographic monitoring to assess implant condition and surrounding tissues for signs of osteolysis.
Here, we present our retrospective assessment of routine clinical and radiographic follow-up data to evaluate the outcomes of the M6-C™ implant. We determined the prevalence, and demographic and clinical risk factors, of implant failure and periprosthetic osteolysis 6–11 years after CTDR with M6-C™.
Methods
Study design
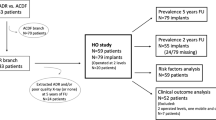
All patients who received an M6-C™ implant between 2011 and 2015 were included (n = 85). Seventeen patients were excluded, including four patients whose implant had been removed for reasons unrelated to osteolysis (Fig. 2). Demographic and surgical data including age, sex, date of surgery, implant level, and the number of levels were reviewed. Ethics approval was granted by the St. John of God Health Care Human Research Ethics Committees and all patients were at least 18 years of age and provided informed consent.
Radiology and grading
Cervical CT scans and anteroposterior X-rays with neutral, flexion, and extension views were thoroughly examined to determine the condition and the stability of the implant, and any periprosthetic bony changes. All CT scans were reviewed by a single experienced orthopaedic spine surgeon (GC). Osteolysis was assessed using the "Osteolysis Grading Scale" (Fig. 3), which classifies endplate involvement. Grades 1 and 2 indicate osteolysis affecting less than 50% of one or both endplates, while Grades 3 and 4 indicate involvement of more than 50% of one or both endplates. The presence of Grade IV HO using the McAfee classification [15] and implant subsidence was also evaluated.
Clinical analyses
Patient-reported outcome measures (PROMs) including the Neck Disability Index (NDI), the Visual Analogue Scale (VAS) for neck and arm pain, and the EQ-5D-5L VAS and Index for health-related quality of life were assessed. Patients reporting a VAS for neck or arm pain intensity greater than 6 or an NDI score greater than 29 were classified as symptomatic. All other patients were categorised as asymptomatic.
Statistical analyses
All analyses were performed using R Statistical Software (R version 4.3.1 (2023-06-16)). Data were assessed and visualised with the ‘ggplot2’ (R Package v0.5.0) and ‘stats’ package in the R environment ("Mountain Hydrangea" Release (547dcf86, 2023-07-07) for Windows) [16, 17]. Data are presented as mean (standard deviation; SD) or median (interquartile range; IQR) as determined by the distribution of the data. Data were assessed using T-tests, Wilcoxon rank-sum tests, Chi-squared tests, Fisher’s exact tests, ANOVA, or Kruskal–Wallis tests as appropriate.
Results
Patient characteristics
We included 36 patients with the M6-C™ implanted at a single level and 7 patients with the M6-C™ at 2 levels who completed follow-up at a mean of 8.1 years (range 6.5–11 years). Demographics and surgical data are shown (Table 1).
Radiographic outcomes
The CT scans of 43 patients were examined, and the 50 implants were graded using the Osteolysis Grading Scale (Fig. 3) and categorised as functional, fused, osteolytic (without implant failure) or failed (with severe osteolysis). Periprosthetic osteolysis with and without implant failure was observed for 22 (44%) implants, and 17 (34%) implants showed a significant degree of HO (McAfee Grade IV; Table 2) [15].
Significant HO resulting in implant fusion was observed in 17 (34%) implants from 9 male and 8 female patients aged [mean (SD)] 44.1 (6.9) years and assessed 8.5 (1.5) years after surgery. Patients with and without fused implants could not be distinguished by their age at surgery (p = 0.90) or gender (p = 0.48). Among the fused implants, 1 (6%) was located at the C3/4 level, 8 (47%) at the C5/6 level, and 8 (47%) at the C6/7 level.
Of the 22 implants exhibiting osteolysis, 17 (34%) were classified as functional (without failure) and 5 (10%; Grades 3–4) as not functional (failed). Implants that had osteolysis but had not failed belonged to 9 female and 7 male patients aged 44.1 (6.9) years and assessed 8.0 (1.4) years after surgery. Most implants with osteolysis were located at the C5/6 (53%) and C6/7 (41%) levels.
The 5 patients with failed implants were aged [mean (SD)] 45.0 (7.3) years and assessed 7.9 (1.5) years after surgery. Notably, all were males (p = 0.016), and the affected implants were all located at the C5/6 level (p = 0.11). There were no differences in age at the time of surgery (p = 0.83), time since surgery (p = 0.85), or the year of surgery (p = 0.75) compared to those with functional, fused or osteolytic implants. Implant failure occurred between 5.4 and 9.0 years after the initial CTDR surgery. One patient underwent initial CTDR in 2013 at C6/7 which had Grade 2 osteolysis after 9 years and a second procedure in 2017 at C5/6 which had subsequently failed with Grade 3 osteolysis after 5 years. Another patient had two single-level procedures, one at the C5/6 level in 2013 that failed with Grade 3 osteolysis after 8 years and a second procedure at the C6/7 level in 2016 which had Grade 1 osteolysis after 6 years. The remaining 3 patients with failed implants had single-level CTDR in 2013 and failed with Grades 3 or 4 osteolysis after 8–9 years. In all cases, revision surgery was performed with the removal of the implant and conversion to fusion. There were no infections observed in any of the revised cases.
Patient reported outcomes
For all patients included in the analysis, the mean (SD) EQ-5D-5L Health VAS and index scores were 77.1 (14.0) and 0.80 (0.17), respectively. The mean (SD) VAS scores for neck and arm pain were 2.8 (2.3) and 1.7 (2.2), respectively. The mean (SD) NDI score was 19.5 (15.4). Compared to patients with no radiographic signs of osteolysis, patients with Grade 4 osteolysis had a significantly lower EQ-5D-5L Health VAS (p = 0.034) and higher VAS for arm pain scores (p = 0.047; Fig. 4).
We investigated the association between PROMs and implant outcomes, namely, failed implants versus implants categorised as functional, fused and osteolytic (without failure; Fig. 5). The EQ-5D-5L Index score and the VAS for neck pain intensity did not differ between implant outcome groups (p > 0.05). However, the EQ-5D-5L Health VAS score was poorer in patients with failed implants compared to those with functional implants (p = 0.029), implants that were fused (p = 0.011) and implants that were osteolytic without failure (p = 0.032; Fig. 5). The NDI score (p = 0.15) and VAS for arm pain intensity (p = 0.15) tended to be poorer in patients with implants in the failed group compared to those with fused implants, but were generally similar to patients with functional or osteolytic implants. Six patients (14%) with a total of seven implants (14%) were categorised as symptomatic (VAS for neck or arm pain intensity greater than 6 or an NDI score greater than 29). Symptomatic patients were a mean age of 47 years, and 4/6 were female. The frequency of implant outcomes was similar between symptomatic and asymptomatic patients (p = 0.65).
Follow-up PROMs scores were compared between implant outcomes; functional, fused, osteolysis (without failure) and failed (with severe osteolysis). a EQ-5D-5L Health VAS, b EQ-5D-5L Index, c VAS for neck pain, d VAS for arm pain, e NDI. EQ-5D-5L Health VAS (a) and VAS for arm pain intensity (d) were significantly poorer for patients with failed implants
Discussion
Periprosthetic osteolysis and high-grade HO were common at a mean follow-up of 8.1 years after CTDR with the M6-C™ implant and were associated with patient-reported outcomes. Among the implants analysed, 22% were classified as normal/functional, while 34% exhibited high-grade HO leading to a fused, immobile implant. Additionally, 44% of the implants showed signs of periprosthetic osteolysis. Notably, 10% of all implants were identified as failed with high grade osteolysis necessitating surgical removal and fusion. All failed implants were found in male patients, and all were located at C5/6.
Grading of periprosthetic osteolysis
It is crucial to differentiate between osteolysis and anterior bone loss. Anterior bone loss is non-inflammatory and related to stress shielding with subsequent bone remodelling that occurs early after arthroplasty but ceases within 6 months of the procedure. Conversely, osteolysis has an inflammatory aetiology triggered by wear, typically manifesting later in the post-operative period [18]. Wahbe et al. introduced a grading system to distinguish between mild, moderate and severe inflammatory osteolysis. However, this grading system lacks explicit values therefore changes over time and between individuals are difficult to assess. The ‘Osteolysis Grading Scale’ used here comprises four grades and provides a simple and comprehensible method to categorise implants based on endplate involvement but has not yet been validated so no inter-rater validity data are available yet. Despite this limitation, we demonstrate a significant association between the severity of osteolysis and patient-reported outcomes. However, given the limited number of implants with Grade 3 and 4 osteolysis, validation studies are warranted to confirm the reliability and robustness of our grading system.
Prevalence of osteolysis in cervical disc total replacement (CDTR)
Periprosthetic osteolysis after CDTR has been described as uncommon [11]. However, a systematic review published in 2020 reported rates of asymptomatic osteolysis ranging from 8 to 64% [19]. While none of the studies described in the systematic review included the M6-C™ implant [19], several reports highlight osteolysis associated with this implant [20,21,22,23,24,25,26,27,28]. In 2022, Scott-Young et al. described 53 patients who received M6-C™ where 34% required revision surgery after an average of 5.5 years, due to osteolysis attributed to a response to the polyethylene components of the M6-C™. The authors concluded that patients with M6-C™ should be proactively contacted, informed and clinically and radiologically evaluated [12]. Nonetheless, this study did not provide indications for revision surgery or report the prevalence of osteolysis or other bony changes that did not necessitate a revision procedure. In our study, 5 patients (12%) required revision surgery due to implant failure with severe osteolysis, which is lower than the reported revision rate of 34%. In 2020, a systematic literature review showed that most cases have only mild or asymptomatic presentations that do not require revision surgery. This may be due to differences in the aetiology of osteolysis as the authors did not distinguish between anterior bone loss and osteolysis [19]. While lower grades of osteolysis may progress over time resulting in increased rates of implant failure, implant failure was not associated with time since the index surgery in our cohort.
Reasons and risk factors for revision surgery of failed implants
The M6-C™ implant is an unconstrained prosthesis featuring PCU outer sheaths, a woven UHMWPE annulus, and PCU nucleus that is intended to closely mimic the natural disc and enhance long-term outcomes and safety (Fig. 1) [14]. Another example of an unconstrained implant design is the Bryan cervical disc (Medtronic Sofamor Danek, Memphis, TN, USA) which also employs an annulus-nucleus analogue and has been associated with osteolysis [19]. Other implants associated with osteolysis include Mobi-C (Zimmer Biomet, USA), Prodisc (Centinel Spine, USA), Discover (DePuy Synthes, USA), and Prestige-SP (Medtronic, USA) [18, 19].
In our study, all failed implants were in male patients with implants at the C5/6 level. Interestingly, out of the 10 male patients in the study with implants at C5/6, 5 implants failed. High failure rate at the C5/6 level is in accord with prior studies [18, 19] and may arise because this segment is among the most mobile in the cervical spine [29]. Our findings suggest that implant failure is not driven by post-operative time or age-related factors.
Heterotopic ossification and fusion after cervical disc total replacement
HO following CDTR was common, affecting 34% of patients in our cohort in accordance with previous studies reporting 4–38% [30]. It is worth noting that fused implants were followed up over a longer time since surgery (p = 0.07) suggesting that the frequency of fused implants may increase over time. Here patients with fused implants exhibited better PROMs than those with functional implants and implants with osteolysis (Fig. 5). Evidence suggests that HO formation does not compromise patient-reported outcomes [31, 32] but radiological studies are lacking. The loss of motion-preservation may lead to increased rates of ASD, as reported with ACDF. On the other hand, the delay to fusion of the implant level due to HO may delay the onset of ASD compared to ACDF. A recent study reported that significant HO after CTDR resulting in immobilised implants did not affect pathology at adjacent segments after 2 or 5-years [31]. As it is difficult to determine when an implant level becomes immobilised, long-term radiographic data are necessary to ascertain the safety profile of CTDR with HO leading to fused implants.
Recommendation for follow-up and future use
We advocate a comprehensive follow-up strategy encompassing radiographic and clinical evaluations for existing patients with an M6-C™ implant. Given the possibility that male patients who have undergone M6-C™ implantation at the C5/6 level may have increased susceptibility to implant failure, a heightened frequency of follow-up appointments should be considered. We also suggest more frequent follow-up for symptomatic patients, particularly those with elevated VAS scores for neck or arm pain intensity and lower NDI scores. This tailored approach will facilitate the early detection of potential complications or concerns, allowing for timely intervention and improved patient well-being.
Considering the rate of fused implants (34%) and the rate of implants with osteolysis (44%), this study brings into question the suitability of this device for future use in the target population, notably younger patients, as it fails to achieve the functional purposes and benefits of CTDR which may lead to greater morbidity for patients. Our institution has discontinued use of the M6-C™ implant based on our study findings however, our results may not reflect the overall performance of the implant. Ongoing review of clinical and radiographic follow-up data will allow treating institutions to determine the suitability of the M6-C™ implant for future use in CTDR.
Limitations
Limitations to our study include the retrospective design and small sample size. The lack of a validated grading system for osteolysis in CDTR may influence the accuracy and consistency of our osteolysis assessments. Additionally, our study did not include a control group, which limits the ability to compare the outcomes of M6-C™ with other CDTR implants or alternative treatment modalities. Multi-centre and prospective studies are warranted to validate our results and assess the safety and efficacy of the M6-C™ implant.
Conclusion
In conclusion, our study highlights a considerable prevalence of periprosthetic osteolysis and fusion associated with the M6-C™ implant in cervical total disc replacement procedures. Implant failure was more prevalent in male patients at the C5/6 level. These findings emphasise the importance of continuous monitoring and surveillance of at-risk patients. Based on these data, we have decided to discontinue the use of the M6-C™ implant at our institution and recommend routine radiographic and clinical follow-up of existing patients. Ongoing research and retrieval analyses will be essential for further elucidating the factors contributing to implant failure and guiding future treatment strategies in cervical disc replacement.
References
Shriver MF, Lewis DJ, Kshettry VR, Rosenbaum BP, Benzel EC, Mroz TE (2015) Pseudoarthrosis rates in anterior cervical discectomy and fusion: a meta-analysis. Spine J 15(9):2016–2027
Matsumoto M, Okada E, Ichihara D, Watanabe K, Chiba K, Toyama Y, Fujiwara H, Momoshima S, Nishiwaki Y, Iwanami A, Ikegami T, Takahata T, Hashimoto T (1976) Anterior cervical decompression and fusion accelerates adjacent segment degeneration: comparison with asymptomatic volunteers in a ten-year magnetic resonance imaging follow-up study. Spine (Phila Pa 1976) 35(1):36–43
Derman PB, Zigler JE (2020) Cervical disc arthroplasty: rationale and history. Int J Spine Surg 14(s2):S5-s13
U.S. Food & Drug Administration Devices. 19 July 2023]; Available from: https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm.
Maayan O, Shafi K, Qureshi S (2023) Update on design and biomechanics of cervical disc arthroplasty. Sem Spine Surg 35(1):101009
Patwardhan AG, Havey RM (2020) Biomechanics of cervical disc arthroplasty—a review of concepts and current technology. Int J Spine Surg 14(s2):S14-s28
The M6-C Artificial Disc Replacement Device—M6-Global. 26 Aug 2022]; Available from: https://m6disc.global/
Byvaltsev VA, Stepanov IA, Riew DK (2020) Mid-term to long-term outcomes after total cervical disk arthroplasty compared with anterior diskectomy and fusion: a systematic review and meta-analysis of randomized controlled trials. Clin Spine Surg 33(5):192–200
Núñez JH, Escudero B, Omiste I, Martínez-Peñas J, Surroca M, Alonzo-González F, Bosch-García D (2023) Outcomes of cervical arthroplasty versus anterior cervical arthrodesis: a systematic review and meta-analysis of randomized clinical trials with a minimum follow-up of 7-year. Eur J Orthop Surg Traumatol 33(5):1875–1884
Wang QL, Tu ZM, Hu P, Kontos F, Li YW, Li L, Dai YL, Lv GH, Wang B (2020) Long-term results comparing cervical disc arthroplasty to anterior cervical discectomy and fusion: a systematic review and meta-analysis of randomized controlled trials. Orthop Surg 12(1):16–30
Parish JM, Asher AM, Coric D (2020) Complications and complication avoidance with cervical total disc replacement. Int J Spine Surg 14(s2):S50-s56
Scott-Young M, Rathbone E, Grierson L (2022) Midterm osteolysis-induced aseptic failure of the M6-C™ cervical total disc replacement secondary to polyethylene wear debris. Eur Spine J 31(5):1273–1282
System for Australian Recall Actions - results. 19 Jul 2023]; Available from: https://apps.tga.gov.au/Prod/sara/arn-report.aspx
M6-C Cervical Artificial Disc Replacement—Orthofix. 24 Mar 2022]; Available from: https://m6disc.com/m6-c-cervical/
McAfee PC, Cunningham BW, Devine J, Williams E, Yu-Yahiro J (2003) Classification of heterotopic ossification (HO) in artificial disk replacement. J Spinal Disord Tech 16(4):384–389
R Core Team (2022) A language and environment for statistical computing. R Foundation for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria
Wickham H (2016) ggplot2: Elegant graphics for data analysis. Springer, New York
Wahbeh JM, Park SH, Campbell P, Ebramzadeh E, Sangiorgio SN (2022) The lexicon for periprosthetic bone loss versus osteolysis after cervical disc arthroplasty: a systematic review. Eur Spine J 31(4):830–842
Joaquim AF, Lee NJ, Lehman RA Jr, Tumialán LM, Riew KD (2020) Osteolysis after cervical disc arthroplasty. Eur Spine J 29(11):2723–2733
Schroven WG, Schroven IT (2020) M6-C prosthesis failure in hybrid construction : a double case report. Acta Orthop Belg 86(4):724–728
Clohisy JCF, Abjornson C, Bauer TW, Baral E, Albert TJ (2023) Delayed failure of M6-C cervical disc arthroplasty after conversion of adjacent cervical disc arthroplasty to fusion: a case report. JBJS Case Connect 13(2):e22
Baltus C, Costa E, Vaz G, Raftopoulos C (2019) Granulomatous reaction on a double-level cervical total disc arthroplasty. World Neurosurg 122:360–363
Roschke E, von der Höh NH, Dietz A, Stingu CS, Gradistanac T, Henkelmann J, Heyde CE (2022) A rare case of wear induced complications after cervical disc replacement. Z Orthop Unfall 160(3):324–328
Brophy CM, Hoh DJ (2018) Compressive cervical pannus formation in a patient after 2-level disc arthroplasty: a rare complication treated with posterior instrumented fusion. J Neurosurg Spine 29(2):130–134
Harati A, Oni P, Oles L, Reuter T, Hamdan M (2020) Vertebral body osteolysis 6 years after cervical disk arthroplasty. J Neurol Surg A Cent Eur Neurosurg 81(2):188–192
Tredan DAM, Mobbs RJ, Maharaj M, Parr WCH (2022) Combining virtual surgical planning and patient-specific 3D-printing as a solution to complex spinal revision surgery. J Pers Med 13(1):19
Harris L, Dyson E, Elliot M, Peterson D, Ulbricht C, Casey A (2019) Delayed periprosthetic collection after cervical disc arthroplasty. J Neurosurg Spine 32(4):1–8
Clark NJ, Francois EL, Freedman BA, Currier B (2020) Early implant failure of a 2-level M6-cervical total disc replacement: a case report. JBJS Case Connect 10(3):e19.00644
Fakhoury J, Dowling TJ (2023) Cervical degenerative disc disease, in StatPearls. StatPearls Publishing, Treasure Island FL
Wahood W, Yolcu YU, Kerezoudis P, Goyal A, Alvi MA, Freedman BA, Bydon M (2020) Artificial discs in cervical disc replacement: a meta-analysis for comparison of long-term outcomes. World Neurosurg 134:598-613.e5
Marques C, MacDowall A, Skeppholm M, Canto Moreira N, Olerud C (2021) Unintended fusion in cervical artificial disk replacement: a prospective study on heterotopic ossification, progression, and clinical outcome, with 5-year follow-up. Eur Spine J 30(6):1662–1669
Hui N, Phan K, Kerferd J, Lee M, Mobbs RJ (2021) Cervical total disc replacement and heterotopic ossification: a review of literature outcomes and biomechanics. Asian Spine J 15(1):127–137
Acknowledgements
The authors thank Patricia Price for reviewing and editing this paper.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This project was supported by LifeHealthCare and Neurospine Foundation. Neurospine Foundation receives industry funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Häckel, S., Gaff, J., Pabbruwe, M. et al. Heterotopic ossification, osteolysis and implant failure following cervical total disc replacement with the M6-C™ artificial disc. Eur Spine J 33, 1292–1299 (2024). https://doi.org/10.1007/s00586-024-08129-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-024-08129-5