Abstract
Purpose
Anterior cervical X-shape-corpectomy and fusion (ACXF) is a novel cervical surgery, designed as partial alternative to the classic technique, anterior cervical corpectomy and fusion (ACCF). The aim of this study was to evaluate the early-stage outcomes of ACXF in treating two-level cervical spondylosis (CS) through comparisons with ACCF.
Methods
A retrospectively comparative study was conducted in two cohorts of patients who underwent single-vertebral ACXF or ACCF to treat two-level CS during September 2019 and October 2021. Clinical and radiological data of all the patients were collected from pre-operation to 1 year after the surgery, following by intra- and intergroup analyses and comparisons.
Results
Fifty-seven patients were included, with 24 undergoing ACXF and 33 undergoing ACCF. ACXF group had significantly shorter drainage duration (2.13 ± 0.61 days vs. 3.48 ± 1.30 days, P < 0.001) and less drainage volume (30.21 ± 26.88 ml vs. 69.30 ± 37.65 ml, P < 0.001) than ACCF group. Both techniques significantly improved all the clinical parameters (P < 0.01) with comparable effects (P > 0.05). Each complication rate in ACXF group was lower than that in ACCF group without significant difference (P > 0.05). ACXF showed a significantly smaller transverse decompression range than ACCF (11.93 ± 1.27 mm vs. 16.29 ± 1.88 mm, P < 0.001). Postoperatively, ACXF yielded a comparable fusion rate (P > 0.05) and a significantly lower subsidence rate (P < 0.01) than ACCF technique at all time points.
Conclusions
ACXF is a potential surgical alternative for certain patients with two-level CS, as it provides both adequate decompression range and fewer adverse events than ACCF. The further modifications on ACXF worth exploration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anterior cervical corpectomy and fusion (ACCF) is a time-tested surgical intervention for patients with cervical spondylosis (CS) that was first described in the 1950s [1]. Multiple clinical trials have demonstrated favorable outcomes with ACCF. In contrast to the classic intervertebral approach, anterior cervical discectomy and fusion (ACDF), ACCF offers a more extensive operative exposure and a broader decompression range, making it applicable to a wider spectrum of indications, particularly in cases where compression extends to the vertebral level [1, 2].
Concomitant with the excellent decompressive effect, however, ACCF is reported to induce more intraoperative and postoperative adverse events. Several systematic reviews have consistently indicated a higher overall complication rate in ACCF compared to ACDF [3, 4]. The extensive corpectomy would inevitably lead to prominent structural disruption and damage, resulting in cerebrospinal fluid (CSF) leakage, haemorrhage and neurological deficit, etc. [5]. Additionally, the commonly used internal implants in ACCF have also been shown to be associated with adverse events. The anterior plate in ACCF may elevate the likelihood of postoperative dysphagia, adjacent segmental degeneration and instrumental failures [6]. And the titanium mesh cage (TMC) has been linked to a higher risk of subsidence [7]. For a subset of patients who present with extensive disc prolapses, localized retrovertebral osteophytes or segmental ossification of posterior longitudinal ligament (OPLL), their decompression requirements may not warrant a traditional corpectomy, while ACCF may expose them to unnecessary risks. To address this concern, we have introduced a novel surgical technique known as anterior X-shape-corpectomy and fusion (ACXF), which combines one-level ACCF and two-level ACDF, serving as a potential alternative for these patients to achieve adequate decompression with a reduced risk of adverse events.
The aim of this study was to assess the early-stage clinical and radiological outcomes of ACXF for treating two-level CS patients by conducting a retrospective comparison between ACXF and ACCF cohorts.
Materials and methods
Study design and participants
A retrospectively comparative study involved two cohorts of consecutive two-level CS patients who aged 40–70 years and had undergone single-level ACXF or ACCF by the same orthopaedic surgeon at our institution between September 2019 and October 2021.
Patients who met any of the following exclusion criterion were excluded: (1) had experienced cervical trauma, tumour, deformation, infection or other operations; (2) had comorbid diseases that would influence the surgical effect, such as severe immunological or metabolic disease; (3) were not follow-up for at least 1 year and (4) did not have adequate clinical and radiological data.
The study was approved by the ethics committee of our institution. Informed consents were obtained from all the patients.
Surgical procedure
The specific procedure of ACXF was described in our previous article [8]. The major procedures included: (1) A standard Smith–Robinson approach was applied to expose the responsible vertebra under the guidance of C-arm fluoroscopy. (2) Conventional intervertebral decompressions were conducted to remove the adjacent discs. (3) A V-shaped corpectomy was implemented on the responsible vertebra using an ultrasonic bone scalpel, the width of which decreased anteriorly to posteriorly with the apex not breaking out the posterior wall of the vertebra. And the resected bone mass was preserved to be reused as a bone graft. (4) An inserted V-shaped corpectomy was performed by ultrasonic scalpel and rongeur to remove the remaining wall along with the compressions. (5) The excised bone mass was trimmed and grafted back into the anterior V-shaped groove. (6) Two appropriate Zero-P or Zero-P VA stand-alone spacers (DePuy Synthes, Massachusetts, USA) were placed into two adjacent intervertebral spaces with screws for fixation. (7) Lastly, the incision was routinely closed (Fig. 1).
Schematic diagram for ACXF surgery. The osteophyte posterior to the C5 body and C4/5, C5/6 disc herniations (a–b); V-shaped corpectomy after C4/5 and C5/6 discectomies (c); inverted V-shaped corpectomy and decompression (d); insertion of the excised and trimmed bone mass (e) and installation of double Zero-P VA systems (f, g)
ACCF was performed in the conventional procedure. The immediately postoperative images of both techniques are presented in Fig. 2.
Clinical evaluation
The perioperative data, including operation time, blood loss, drainage duration and volume, were recorded. Patient-reported outcomes, including visual analogue scale score for the neck (VASneck), neck disability index (NDI) and Japanese Orthopaedic Association (JOA) score, were utilized for clinical assessment [9]. The recovery rate (RR) was calculated as: (postoperative JOA—preoperative JOA)/(17—preoperative JOA) × 100%. All the clinical parameters were investigated preoperatively and at routine intervals of 1 day, 3 months, 6 months and 1 year postoperatively. In addition, any intraoperative or postoperative complication was detected and recorded in detail.
Radiological evaluation
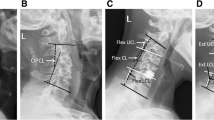
All patients had taken radiological examinations of X-ray, computed tomography (CT) and magnetic resonance imaging (MRI) preoperatively and at routine intervals of 1 day, 3 months, 6 months and 1 year postoperatively. The images were collected to evaluate the radiological parameters, including cervical lordosis (CL), sagittal vertical axis (SVA), T1 slope (T1S), functional spinal unit (FSU) height, FSU range of motion (ROM), C2–C7 ROM, prevertebral soft tissue thickness (PSTT), fusion rate, subsidence rate, radiological adjacent segment pathology (RASP), transverse decompression range (TDR), anteroposterior canal diameter (APCD) of the spinal canal and spinal canal area (SCA). On lateral radiographs, CL, SVA, T1S, FSU height, FSU ROM and C2-C7 ROM were assessed according to conventional definitions. PSTT was determined as the length of a line from posterior aspect of the trachea to the centre of the anterior vertebral cortex, which was paralleled to the upper endplate [10]. In each case, the average PSTT of two vertebral levels adjacent to the responsible vertebra was taken into analysis. And ΔPSTT was the difference of PSTT before and after the surgery. Solid bony fusion was defined as the presence of a new trabecular bony bridge or FSU ROM observed from dynamic lateral radiographs was less than 2° [11]. Subsidence was defined as over 2-mm loss of FSU height compared with that of the 1 day postoperative radiograph [12]. The RASP was assessed preoperatively and 1 year postoperatively according to the criteria established by Kellgren et al., which involves at least one of the following signs on radiographs: (1) anterior osteophytosis or ossification of anterior longitudinal ligament, (2) loss of disc height and (3) sclerosis of vertebral endplate [13]. The newly onset or progression of RASP from pre-operation to 1 year post-operation was recorded. TDR, APCD and SCA were measured on cross-sectional CT. Measurements concerning part of parameters are illustrated in Fig. 3.
Statistical analysis
The quantitative data were shown as mean ± standard deviation (normally distributed) or median (interquartile range) (not normally distributed), while the qualitative data were expressed as fractions. For quantitative data, paired t-test or Wilcoxon signed-rank test was used for intragroup comparisons at different time points, and an independent t-test or Mann‒Whitney U-test was utilized for intergroup comparisons. The Pearson X2 test or Fisher precision probability test was adopted for the analysis of qualitative data. All the data were analysed using IBM SPSS Statistics, version 26.0 (IBM Corp., Armonk, New York, USA). All reported P values were two-sided, and the difference was considered to be statistically significant when the P value < 0.05.
Results
Baseline data
A total of 57 patients were enrolled in the study, among whom 24 underwent ACXF and 33 underwent ACCF. The specific values of the baseline data of two groups are displayed in Table 1. There were no significant differences detected in any baseline data between two groups (P > 0.05), except in follow-up time (13.92±2.59 months vs. 15.64±3.39 months, P = 0.042).
Clinical results
In average, ACXF group had shorter operation time (143.75 ± 29.31 mins vs. 155.45 ± 27.51 mins, P = 0.129) and less blood loss (106.67 ± 47.24 ml vs. 127.88 ± 48.59 ml, P = 0.105) than ACCF group without significances. Drainage duration (2.13 ± 0.61 days vs. 3.48 ± 1.30 days, P < 0.001) and drainage volume (30.21 ± 26.88 ml vs. 69.30 ± 37.65 ml, P < 0.001) were significantly less in ACXF group than that in ACCF group. JOA, NDI and VASneck scores significantly improved in both groups compared to those before the operation (P < 0.01). No significant intergroup differences were observed in these clinical parameters and RR rate at almost all time points (P > 0.05). In ACXF group, there were two cases of dysphagia and one case of hoarseness, who were alleviated after several days of appropriate treatment. While in ACCF group, there were six cases of dysphagia, four cases of CSF leakage, three cases of hoarseness and one case of haematoma. Compared to ACCF group, ACXF group yielded relatively lower incidences for each complication, but the intergroup differences were not significant (P > 0.05). None of other complications were observed in either group (Table 2).
Radiological results
CL improved after both surgeries, with no significant intergroup differences observed in CL, SVA and T1S at any time point (P > 0.05). FSU height for both groups peaked immediately after the surgery, but gradually decreased during the follow-up period. Postoperative FSU height loss was greater in ACCF group compared to ACXF group (Fig. 4). ACXF yielded a significantly smaller TDR than ACCF (11.93 ± 1.27 mm vs. 16.29 ± 1.88 mm, P < 0.001). APCD and SCA were significantly enlarged after both techniques (P < 0.01), while postoperative SCA for ACXF group was significantly smaller than that for ACCF group (P ≤ 0.001). PSTT also increased significantly (P < 0.01) after both surgeries. There were significant differences between ACXF and ACCF groups in postoperative PSTT (18.02 ± 3.27 mm vs. 20.80 ± 3.79 mm, P = 0.005) and ΔPSTT (6.38 ± 2.59 mm vs. 10.15 ± 2.91 mm, P < 0.001). Fusion rate was consistently comparable between two groups (P > 0.05), while subsidence rate was significantly lower in ACXF group than that in ACCF group at all postoperative time points (P < 0.01). None of significant differences were detected in general, newly-onset or progressive RASP between two groups 1 year after the surgery (P > 0.05) (Table 3). A typical case is shown in Fig. 5.
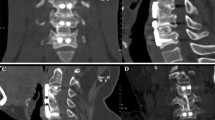
A 48-year-old male who complained about muscle weakness of both upper extremities for 2 years with aggravation and difficulty walking for 3 months was diagnosed with cervical spondylotic myelopathy. The patient received ACXF on C5 vertebra, whose symptoms were alleviated immediately after the surgery. He recovered smoothly without any complication occurred and was discharged 8 days after admission. Preoperative images a–g showed OPLL at C5 level (a, b), disc prolapses at C4/5 and C5/6 levels (c, d), leading to compressions on spinal cord (e–g); immediately postoperative images h–n showed that the bony graft and Zero-P VA spacers were in appropriate positions (h). The compressions were totally removed, and spinal canal was enlarged (i–k), providing adequate space for spinal cord with continuous CSF signal emerging (l–n); 6-month postoperative images o–t showed that the internal fixations stayed steady in neutral, flexion and extension positions (o–q). Bridging trabecular bone at adjacent intervertebral levels was detected on sagittal sections (r). Transverse sections showed apparent fusion between bony graft and bilateral residual vertebral walls (s, t)
Discussion
This study evaluated the clinical and radiological outcomes of a novel surgical technique, ACXF, and compared them with those of ACCF in the management of patients with two-level CS. ACXF was originally designed for CS patients with extensive disc prolapses, localized retrovertebral osteophytes or segmental OPLL, who required an intermediate decompression range between that provided by ACCF and ACDF. As ACXF is considered as partial alternative to ACCF, it is important to determine its optimal indications. The current research indicated a gratifying clinical efficacy of ACXF for cases involving compression with a transverse dimension less than 12 mm. All patients exhibited a significant increase in APCD and SCA, rendering sufficient room to release the spinal cord. Correspondingly, their clinical parameters improved as the neurological symptoms were relieved, which was comparable to the outcomes observed after ACCF. However, for patients presenting with extremely lateral disc prolapses, osteophytes or wide-based OPLL, the priority of ACXF may diminish.
ACXF showed superiority in minimalize the surgical trauma. The foundation of ACXF lies in the two-step corpectomy, comprising an initial V-shaped corpectomy followed by an inverted V-shaped corpectomy, combinedly forming an X-shaped profile in the transverse view of the vertebra. The first “V” groove facilitates the exposure of the operative field, while the second “inverted V” groove enhances direct anterior decompression. This two-step corpectomy contributes to the preservation of the responsible vertebra, in stark contrast to conventional corpectomy which covers majority of the vertebral body and exposes nearby anatomical structures to potential injuries, increasing the risks of haemorrhage, CSF leakage, infection and neurological deficit [14, 15]. In ACXF, guided by preoperative and intraoperative imaging, we limited the bone resection within the X-shaped groove, reducing disruption and damage to surrounding tissues, especially to the nerves and dura mater. In this study, ACXF resulted in relatively less blood loss, significantly shorter drainage durations and less volumes. There were no cases of CSF leakage, haematoma or neurological deficit, and only one case of hoarseness detected among the ACXF cohort, indicating the satisfactory safety of ACXF.
The implants used in ACXF differed from those in conventional ACCF, which typically incorporates anterior plate and TMC. While anterior platerender robust fixation, it could elevate the risk of dysphagia [16]. In ACXF, two Zero-P (VA) spacers are employed in lieu of the plate to maintain the intervertebral height and provide internal fixation. Previous articles had compared the both implants and concluded that Zero-P had better performance than the plate in mitigating dysphagia [17, 18]. Theoretically, dysphagia following anterior cervical surgery is mainly attributed to the impact on prevertebral structures, which leads to soft tissue swelling and irritation of the oesophagus [19]. ΔPSTT was adopted to quantify the influence on the prevertebral tissues through the surgery, which had shown a significantly positive correlation with dysphagia [20]. The data that ACXF exhibited significantly smaller ΔPSTT than ACCF reflected the optimized prevertebral disturbance of the low-profile design of Zero-P (VA). Consequently, ACXF group experienced a relatively lower dysphagia rate, with only one patient affected and promptly alleviated after appropriate conservative treatment.
Bony fusion and subsidence represent two critical parameters directly linked to surgical outcomes. The conventional use of TMC in ACCF has been praised for satisfactory fusion effect. Nevertheless, the TMC had been noticed with a high rate of subsidence, which could lead to cervical instability, recurrent neurological deteriorations, implant failure and even necessitate revision surgery [21]. Chen et al. reviewed 300 ACCF cases with TMC and reported that 79.7% of cases were observed with reduction in FSU height during 1 year [22]. The potential factors contributing to the elevated incidence of subsidence with TMC may include stress concentration resulting from the mismatch between TMC and endplate interfaces, as well as the high stiffness of TMC and excessive endplate grinding during insertion [23, 24]. In ACXF, we apply in situ bone graft instead of TMC, where we trim and reinsert the V-shaped autologous bone mass from local vertebrae, thereby preserving structural integrity. Based on our experience, the graft remains spontaneously stable without the need for additional fixation under longitudinal pressure and extrusion from bilateral inclined bony surfaces. The use of in situ bone graft in combination with the Zero-P (VA) systems enables even interface contact, significantly reducing stress concentration and creating a stable biomechanical environment. Accordingly, our data demonstrated better maintenance of FSU height 1 year after ACXF and confirmed significantly lower subsidence rates of ACXF at all time points compared to contemporary data from ACCF with TMC, as reported by us and previous studies [12, 25]. Furthermore, the bone graft exposes sufficient bone marrow and allows direct cancellous–cancellous bone contact with the residual vertebra. This facilitates the crawling of new bone without obstruction from cortical bone. Consequently, ACXF group achieved a satisfying fusion rate in the early stages and 1 year after the surgery. Besides, in situ graft approach could substitute iliac bone harvesting and eliminate the risk of donor-site complications.
In other aspects, ACXF group showed comparable efficacy in reconstructing the cervical lordosis and sagittal alignment in comparison with ACCF group. Short-term RASP did not reveal any significant intergroup difference. The above parameters warrant close monitoring during the medium- to long-term follow-up assessments.
It is noteworthy that the adoption of ACXF should be considered with caution for patients with dural adhesions, as the direct separation of the adherent posterior longitudinal ligament and dura sac from an anterior approach carries a significant risk of CSF leakage. Additionally, ACXF is a relatively intricate and technically demanding procedure, especially with decompression after two-step corpectomy, which may pose challenges for grasping. Lastly, during installation of the Zero-P (VA) systems, there exists a risk of screws crossing the graft–vertebra interface and drilling into the graft, which could compromise the holding force of screws and graft vascularization, and further affect the holistic stability of internal fixation and fusion outcome. A modified and dedicated internal fixation system for ACXF technique might be the ultimate solution. Despite these limitations, our study underscored the inspiring outcomes and reduced incidence of adverse events with ACXF. Therefore, it is suggested as a viable surgical alternative for select two-level CS patients, meriting further in-depth investigation.
Some limitations of this study should be emphasized. First, concerning that ACXF was still in its infancy, this study was designed as a non-randomized, single-centre retrospective comparison with a small sample size and short follow-up time, thus some confounders were unavoidable and the level of evidence was low. A multicentre prospective randomized comparison study with a large sample and long-term follow-up should be conducted in the future to corroborate the long-term outcomes and reliability of ACXF. Moreover, further biomechanical and basic research is needed to elucidate the fate of reinserted V-shaped autologous bone mass and its impact on the biomechanical environment of the responsible vertebra and adjacent segments.
Conclusion
ACXF presents a promising surgical option for two-level CS patients with extensive disc prolapses, localized retrovertebral osteophytes or segmental OPLL and with compression width less than 12 mm.It could achieve sufficient decompression and simultaneously mitigate the occurrence of adverse events. Nonetheless, there are some inherent limitations with ACXF that necessitate future improvement and modification.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACCF:
-
Anterior cervical corpectomy and fusion
- CS:
-
Cervical spondylosis
- ACDF:
-
Anterior cervical discectomy and fusion
- CSF:
-
Cerebrospinal fluid
- TMC:
-
Titanium mesh cage
- OPLL:
-
Ossification of posterior longitudinal ligament
- ACXF:
-
Anterior X-shape-corpectomy and fusion
- VASneck:
-
Visual analogue scale score for the neck
- NDI:
-
Neck disability index
- JOA:
-
Japanese Orthopaedic Association
- RR:
-
Recovery rate
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- CL:
-
Cervical lordosis
- SVA:
-
Sagittal vertical axis
- T1S:
-
T1 slope
- FSU:
-
Functional spinal unit
- ROM:
-
Range of motion
- TDR:
-
Transverse decompression range
- APCD:
-
Anteroposterior canal diameter
- PSTT:
-
Prevertebral soft tissue thickness
- SCA:
-
Spinal canal area
- RASP:
-
Radiological adjacent segment pathology
References
Louie PK, Nemani VM, Leveque J-CA (2022) Anterior cervical corpectomy and fusion for degenerative cervical spondylotic myelopathy: case presentation with surgical technique demonstration and review of literature. Clin Spine Surg 35:440–446. https://doi.org/10.1097/BSD.0000000000001410
Ghogawala Z (2018) Anterior cervical option to manage degenerative cervical myelopathy. Neurosurg Clin N Am 29:83–89. https://doi.org/10.1016/j.nec.2017.09.005
Wang T, Wang H, Liu S et al (2016) anterior cervical discectomy and fusion versus anterior cervical corpectomy and fusion in multilevel cervical spondylotic myelopathy: a meta-analysis. Med Baltim 95:e5437. https://doi.org/10.1097/MD.0000000000005437
Yee TJ, Swong K, Park P (2020) Complications of anterior cervical spine surgery: a systematic review of the literature. J Spine Surg Hong Kong 6:302–322. https://doi.org/10.21037/jss.2020.01.14
Zhang Y, Liu H, Yang H, Pi B (2018) Anterior cervical corpectomy and fusion versus discectomy and fusion for the treatment of two-level cervical spondylotic myelopathy: analysis of sagittal balance and axial symptoms. Int Orthop 42:1877–1882. https://doi.org/10.1007/s00264-018-3804-3
Zavras AG, Nolte MT, Sayari AJ et al (2022) Stand-alone cage versus anterior plating for 1-level and 2-level anterior cervical discectomy and fusion: a randomized controlled trial. Clin Spine Surg 35:155–165. https://doi.org/10.1097/BSD.0000000000001332
Ji C, Yu S, Yan N et al (2020) Risk factors for subsidence of titanium mesh cage following single-level anterior cervical corpectomy and fusion. BMC Musculoskelet Disord 21:32. https://doi.org/10.1186/s12891-019-3036-8
Liu Y, Meng Y, Liu H et al (2021) A novel anterior cervical x-shape-corpectomy and fusion for cervical spinal stenosis at c4–c6 level: a technical note. World Neurosurg 149:181–189. https://doi.org/10.1016/j.wneu.2021.02.098
Weinfurt KP, Reeve BB (2022) Patient-reported outcome measures in clinical research. JAMA 328:472–473. https://doi.org/10.1001/jama.2022.11238
Suk K-S, Kim K-T, Lee S-H, Park S-W (2006) Prevertebral soft tissue swelling after anterior cervical discectomy and fusion with plate fixation. Int Orthop 30:290–294. https://doi.org/10.1007/s00264-005-0072-9
Obermueller T, Wagner A, Kogler L et al (2020) Radiographic measurements of cervical alignment, fusion and subsidence after ACDF surgery and their impact on clinical outcome. Acta Neurochir Wien 162:89–99. https://doi.org/10.1007/s00701-019-04139-1
Tang Y, Geng X, Li F et al (2022) Factors affecting titanium mesh cage subsidence in single-level anterior cervical corpectomy and fusion for ossification of the posterior longitudinal ligament. J Orthop Surg 17:515. https://doi.org/10.1186/s13018-022-03409-6
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16:494–502. https://doi.org/10.1136/ard.16.4.494
Lin Q, Zhou X, Wang X et al (2012) A comparison of anterior cervical discectomy and corpectomy in patients with multilevel cervical spondylotic myelopathy. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 21:474–481. https://doi.org/10.1007/s00586-011-1961-9
Yu Z, Shi X, Yin J et al (2022) Comparison of complications between anterior cervical diskectomy and fusion versus anterior cervical corpectomy and fusion in two- and three-level cervical spondylotic myelopathy: a meta-analysis. J Neurol Surg Part Cent Eur Neurosurg. https://doi.org/10.1055/s-0042-1747926
Liu J, Hai Y, Kang N et al (2018) Risk factors and preventative measures of early and persistent dysphagia after anterior cervical spine surgery: a systematic review. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 27:1209–1218. https://doi.org/10.1007/s00586-017-5311-4
Yin M, Ma J, Huang Q et al (2016) The new Zero-P implant can effectively reduce the risk of postoperative dysphagia and complications compared with the traditional anterior cage and plate: a systematic review and meta-analysis. BMC Musculoskelet Disord 17:430. https://doi.org/10.1186/s12891-016-1274-6
Zhang T, Guo N, Gao G et al (2022) Comparison of outcomes between Zero-p implant and anterior cervical plate interbody fusion systems for anterior cervical decompression and fusion: a systematic review and meta-analysis of randomized controlled trials. J Orthop Surg 17:47. https://doi.org/10.1186/s13018-022-02940-w
Kepler CK, Rihn JA, Bennett JD et al (2012) Dysphagia and soft-tissue swelling after anterior cervical surgery: a radiographic analysis. Spine J Off J North Am Spine Soc 12:639–644. https://doi.org/10.1016/j.spinee.2012.03.024
Shi S, Li X-F, Zhao Q-T et al (2016) Risk factors for dysphagia after single-level anterior cervical decompression with arthroplasty or fusion: a prospective study comparing 2 zero-profile implants. World Neurosurg 95:148–155. https://doi.org/10.1016/j.wneu.2016.07.100
Wen Z, Lu T, Wang Y et al (2018) Anterior Cervical Corpectomy and Fusion and Anterior Cervical Discectomy and Fusion Using Titanium Mesh Cages for Treatment of Degenerative Cervical Pathologies: A Literature Review. Med Sci Monit Int Med J Exp Clin Res 24:6398–6404. https://doi.org/10.12659/MSM.910269
Chen Y, Chen D, Guo Y et al (2008) Subsidence of titanium mesh cage: a study based on 300 cases. J Spinal Disord Tech 21:489–492. https://doi.org/10.1097/BSD.0b013e318158de22
Wu J, Luo D, Ye X et al (2015) Anatomy-related risk factors for the subsidence of titanium mesh cage in cervical reconstruction after one-level corpectomy. Int J Clin Exp Med 8:7405–7411
Weber MH, Fortin M, Shen J et al (2017) Graft subsidence and revision rates following anterior cervical corpectomy: a clinical study comparing different interbody cages. Clin Spine Surg 30:E1239–E1245. https://doi.org/10.1097/BSD.0000000000000428
Lau D, Song Y, Guan Z et al (2013) Radiological outcomes of static vs expandable titanium cages after corpectomy: a retrospective cohort analysis of subsidence. Neurosurgery 72:529–539. https://doi.org/10.1227/NEU.0b013e318282a558.
Funding
This study was funded by the National Natural Science Foundation of China [grant no. 82172522 to Hao Liu], the Cadre Health Research Project of Sichuan Province [grant no. ZH2023-105 to Hao Liu] and the Clinical Research Incubation Project of West China Hospital of Sichuan University [grant no. 2022HXFHO17 to Chen Ding], the National Natural Science Foundation of China [grant no. 82302785 to Tingkui Wu].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design. Concepts and funding sources were provided by [HL], CD and [TW]. Material preparation, data collection and analysis were performed by [HW], [YL] and [XR]. The first draft of the manuscript was written by [HW], [YL] and [TW] and reviewed by [KH], [HL] and [BW]. The first revision of the draft was conducted by [HW], [CY] and [JH]. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of West China Hospital, Sichuan University (No. 2022(305)).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 5.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, H., Liu, Y., Wu, T. et al. Anterior cervical X-shape-corpectomy and fusion vs. anterior cervical corpectomy and fusion for two-level cervical spondylosis. Eur Spine J 33, 205–215 (2024). https://doi.org/10.1007/s00586-023-07986-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07986-w