Abstract
Purpose
Among the many questionnaires available to evaluate low back pain (LBP) patients, the Core Outcome Measures Index (COMI) has the unique advantage to investigate five dimensions using seven short questions. The aim of this study was to explore additional properties of the questionnaire in a French-speaking non-surgical population.
Methods
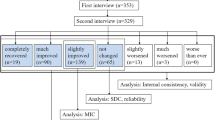
This study was conducted on 168 patients suffering from subacute or chronic LBP and followed up for 6 months in three French-speaking countries. In addition to basic psychometric properties (e.g., construct validity, floor and ceiling effect, reproducibility), internal validity was analyzed by a factor analysis using Cronbach’s alpha. Responsiveness and sensitivity to change were assessed through minimal detectable change (MDC), effect size, and Minimal Clinically Important Improvement (MCII). We used an anchor-based method with receiver operating characteristic (ROC) curve analysis to assess MCII and the Patient Acceptable Symptom State.
Results
Construct validity, reliability (Cronbach’s alpha = 0.87), reproducibility and the absence of floor and ceiling effects were confirmed. Factor analysis indicated a one-dimensional construct that validates the use of a sum score. The MDC (2.1) was inferior to the MCII (2.3). The limit below which the patient claims to be in a fair condition (Patient Acceptable Symptom State) was set at 3.
Conclusions
The COMI is a self-report questionnaire with the capacity to easily and quickly explore several dimensions in patients with LBP that can be then summarized in a meaningful sum score. Additional knowledge provided by our study should encourage the widespread use of the COMI among the spine community.


Similar content being viewed by others
References
Cleland JJ, Gillani RR, Bienen EJR, Sadosky AA (2011) Assessing dimensionality and responsiveness of outcomes measures for patients with low back pain. Pain pract 11(1):57–69
Deyo RA, Battie M, Beurskens AJ, Bombardier C, Croft P, Koes B, Malmivaara A, Roland M, Von Korff M, Waddell G (1998) Outcome measures for low back pain research. A proposal for standardized use. Spine 23(18):2003–2013
Ferrer M, Pellise F, Escudero O, Alvarez L, Pont A, Alonso J, Deyo R (2006) Validation of a minimum outcome core set in the evaluation of patients with back pain. Spine 31(12):1372–1379 discussion 1380
Genevay S, Cedraschi C, Marty M, Rozenberg S, De-Goumons P, Faundez A, Balagu F, Porchet F, Mannion AFA (2012) Reliability and validity of the cross-culturally adapted French version of the core outcome measures index (COMI) in patients with low back pain. Eur Spine J 21(1):130–137
Mannion AF, Elfering A, Staerkle R, Junge A, Grob D, Semmer NK, Jacobshagen N, Dvorak J, Boos N (2005) Outcome assessment in low back pain: how low can you go? Eur Spine J 14(10):1014–1026
Roeder C, Chavanne A, Mannion AF, Grob D, Aebi M (2005) SSE Spine Tango–content, workflow, set-up. www.eurospine.org-Spine Tango. Eur Spine J 14(10):920–924
Kvien TK, Heiberg T, Hagen KB (2007) Minimal clinically important improvement/difference (MCII/MCID) and patient acceptable symptom state (PASS): what do these concepts mean? Ann Rheum Dis 66(Suppl 3):iii40–iii41
Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, Bouter LM, de Vet HC (2007) Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 60(1):34–42
Storheim K, Brox JI, Lochting I, Werner EL, Grotle M (2012) Cross-cultural adaptation and validation of the Norwegian version of the Core Outcome Measures Index for low back pain. Eur Spine J 21(12):2539–2549
Coste J, Le Parc JM, Berge E, Delecoeuillerie G, Paolaggi JB (1993) French validation of a disability rating scale for the evaluation of low back pain (EIFEL questionnaire). Rev Rhum Ed Fr 60(5):335–341
Marty M, Blotman F, Avouac B, Rozenberg S, Valat JP (1998) Validation of the French version of the Dallas Pain Questionnaire in chronic low back pain patients. Rev Rhum Engl Ed 65(2):126–134
Perneger TV, Combescure C, Courvoisier DS (2010) General population reference values for the French version of the EuroQol EQ-5D health utility instrument. Value Health 13(5):631–635
Beurskens AJ, de Vet HC, Koke AJ (1996) Responsiveness of functional status in low back pain: a comparison of different instruments. Pain 65(1):71–76
Tubach F, Ravaud P, Beaton D, Boers M, Bombardier C, Felson DT, van der Heijde D, Wells G, Dougados M (2007) Minimal clinically important improvement and patient acceptable symptom state for subjective outcome measures in rheumatic disorders. J Rheumatol 34(5):1188–1193
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum Associates, Hillsdale
Steiner D, Norman G (1995) Health measurement scales: a practical guide to their development and use. Oxford Medical Publications, Oxford
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476):307–310
Hopkins WG (2000) Measures of reliability in sports medicine and science. Sports med 30(1):1–15
Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, Bombardier C, Felson D, Hochberg M, van der Heijde D, Dougados M (2005) Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis 64(1):29–33
Tubach F, Wells GA, Ravaud P, Dougados M (2005) Minimal clinically important difference, low disease activity state, and patient acceptable symptom state: methodological issues. J Rheumatol 32(10):2025–2029
Qiao J, Zhu F, Zhu Z, Xu L, Wang B, Yu Y, Qian BP, Ding Y, Qiu Y (2013) Validation of the simplified Chinese version of the Core Outcome Measures Index (COMI). Eur Spine J 22(12):2821–2826
Escobar A, Gonzalez M, Quintana JM, Vrotsou K, Bilbao A, Herrera-Espineira C, Garcia-Perez L, Aizpuru F, Sarasqueta C (2012) Patient acceptable symptom state and OMERACT-OARSI set of responder criteria in joint replacement. Identification of cut-off values. Osteoarthr Cartil 20(2):87–92
Tubach F, Dougados M, Falissard B, Baron G, Logeart I, Ravaud P (2006) Feeling good rather than feeling better matters more to patients. Arthritis Rheum 55(4):526–530
Tubach F, Pham T, Skomsvoll JF, Mikkelsen K, Bjorneboe O, Ravaud P, Dougados M, Kvien TK (2006) Stability of the patient acceptable symptomatic state over time in outcome criteria in ankylosing spondylitis. Arthritis Rheum 55(6):960–963
Hochberg MCM, Wohlreich MM, Gaynor PP, Hanna SS, Risser RR (2011) Clinically relevant outcomes based on analysis of pooled aata from 2 trials of Duloxetine in patients with knee Osteoarthritis. J Rheumatol 39(2):352–358
Quintana JMJ, Aguirre UU, Barrio II, Orive MM, Garcia SS, Escobar AA (2011) Outcomes after total hip replacement based on patients’ basal status, what results you can expect. Arthritis Care Res (Hoboken) 64(4):563–572
Mannion AF, Porchet F, Kleinstuck FS, Lattig F, Jeszenszky D, Bartanusz V, Dvorak J, Grob D (2009) The quality of spine surgery from the patient’s perspective: part 2. Minimal clinically important difference for improvement and deterioration as measured with the Core Outcome Measures Index. Eur Spine J 18(Suppl 3):374–379
Mannion AF, Boneschi M, Teli M, Luca A, Zaina F, Negrini S, Schulz PJ (2012) Reliability and validity of the cross-culturally adapted Italian version of the Core Outcome Measures Index. Eur Spine J 21(Suppl 6):S737–S749
Miekisiak G, Banach M, Kiwic G, Kubaszewski L, Kaczmarczyk J, Sulewski A, Kloc W, Libionka W, Latka D, Kollataj M, Zaluski R (2014) Reliability and validity of the Polish version of the Core Outcome Measures Index for the neck. Eur Spine J 23(4):898–903
Acknowledgments
We thank all the team from “Nukleus” and, in particular, Mrs V. Gordin and M. Demonnet for their help and logistic support. We wish also to thank members of the Spine section of the French Rheumatology Society for their support in recruiting patients and Pfizer AG for their financial support. This study was supported by an unrestricted educational grant from Pfizer.
Conflict of interest
None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Genevay, S., Marty, M., Courvoisier, D.S. et al. Validity of the French version of the Core Outcome Measures Index for low back pain patients: a prospective cohort study. Eur Spine J 23, 2097–2104 (2014). https://doi.org/10.1007/s00586-014-3325-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-014-3325-8