Abstract
Purpose
The pathomechanisms of pain resulting from lumbar disc herniation have not been fully elucidated. Prostaglandins and cytokines generated at the inflammatory site produce associated pain; however, non-steroidal anti-inflammatory drugs and steroids are sometimes ineffective in patients. Tetrodotoxin-sensitive voltage-gated sodium (NaV) channels are related to sensory transmission in primary sensory nerves. The sodium channel NaV1.7 has emerged as an attractive analgesic target. The purpose of this study was to evaluate pain-related behavior and expression of NaV1.7 in dorsal root ganglia (DRG) after combined sciatic nerve compression and nucleus pulposus (NP) application in rats.
Methods
Rats were divided into three groups and underwent either sciatic nerve compression with NP for 2 s using forceps (n = 20), sham operation with neither compression nor NP (n = 20), or no operation (controls, n = 20). Mechanical hyperalgesia was measured every second day for three weeks using von Frey filaments. NaV1.7 expression in L5 DRG was examined 7 and 14 days after surgery using immunohistochemistry. The number of neurons immunoreactive for NaV1.7 was compared among the three groups.
Results
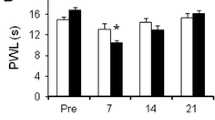
Mechanical hyperalgesia was found over the 14-day observation in the nerve compression plus NP application group, but not in the sham-operated or control groups (P < 0.05). NaV1.7 expression in L5 DRG was up-regulated in the nerve compression plus NP application group, compared with sham-operated and control rats (P < 0.01).
Conclusions
Our results indicate that nerve compression plus NP application produces pain-related behavior. We conclude that NaV1.7 expression in DRG neurons may play an important role in mediating pain from sciatic nerves after compression injury and exposure to NP.



Similar content being viewed by others
References
Olmarker K, Rydevik B, Nordborg C (1993) Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine 18:132–1425
Olmarker K, Larsson K (1998) Tumor necrosis factor alpha and nucleus-pulposus-induced nerve root injury. Spine 23:2538–2544
Toyone T, Takahashi K, Kitahara H, Yamagata M, Murakami M, Moriya H (1993) Visualisation of symptomatic nerve roots. Prospective study of contrast-enhanced MRI in patients with lumbar disc herniation. J Bone Joint Surg Br 75:529–533
Olmarker K, Storkson R, Berge OG (2002) Pathogenesis of sciatic pain: a study of spontaneous behavior in rats exposed to experimental disc herniation. Spine 27:1312–1317
Olmarker K, Nutu M, Storkson R (2003) Changes in spontaneous behavior in rats exposed to experimental disc herniation are blocked by selective TNF-alpha inhibition. Spine 28:1635–1641
Hatori M, Kokubun S (1999) Clinical use of etodolac for the treatment of lumbar disc herniation. Curr Med Res Opin 15:193–201
Ito T, Ohtori S, Inoue G, Koshi T, Doya H, Saito T, Moriya H, Takahashi K (2007) Glial phosphorylated p38 MAP kinase mediates pain in a rat model of lumbar disc herniation and induces motor dysfunction in a rat model of lumbar spinal canal stenosis. Spine 32:159–167
Cohen SP, Bogduk N, Dragovich A, Buckenmaier CC 3rd, Griffith S, Kurihara C, Raymond J, Richter PJ, Williams N, Yaksh TL (2009) Randomized, double-blind, placebo-controlled, dose-response, and preclinical safety study of transforaminal epidural etanercept for the treatment of sciatica. Anesthesiology 110:1116–1126
Korhonen T, Karppinen J, Malmivaara A, Autio R, Niinimäki J, Paimela L, Kyllönen E, Lindgren KA, Tervonen O, Seitsalo S, Hurri H (2004) Efficacy of infliximab for disc herniation-induced sciatica: one-year follow-up. Spine 29:2115–2119
Korhonen T, Karppinen J, Paimela L, Malmivaara A, Lindgren KA, Järvinen S, Niinimäki J, Veeger N, Seitsalo S, Hurri H (2005) The treatment of disc herniation-induced sciatica with infliximab: results of a randomized, controlled, 3-month follow-up study. Spine 30:2724–2728
Korhonen T, Karppinen J, Paimela L, Malmivaara A, Lindgren KA, Bowman C, Hammond A, Kirkham B, Järvinen S, Niinimäki J, Veeger N, Haapea M, Torkki M, Tervonen O, Seitsalo S, Hurri H (2006) The treatment of disc-herniation-induced sciatica with infliximab: one-year follow-up results of FIRST II, a randomized controlled trial. Spine 31:2759–2766
Rupasinghe DB, Knapp O, Blomster LV, Schmid AB, Adams DJ, King GF, Ruitenberg MJ (2012) Localization of Nav 1.7 in the normal and injured rodent olfactory system indicates a critical role in olfaction, pheromone sensing and immune function. Channels (Austin):103–10
King GF, Escoubas P, Nicholson GM (2008) Peptide toxins that selectively target insect Na(V) and Ca(V) channels. Channels (Austin) 2:100–16. PMID:18849658. doi:10.4161/chan.2.2.6022
Dib-Hajj SD, Cummins TR, Black JA, Waxman SG (2007) From genes to pain: Na v 1.7 and human pain disorders. Trends Neurosci 30:555–563
Estacion M, Harty TP, Choi JS, Tyrrell L, Dib-Hajj SD, Waxman SG (2009) A sodium channel gene SCN9A polymorphism that increases nociceptor excitability. Ann Neurol. 66:862–866
Waxman SG, Dib-Hajj S (2005) Erythermalgia: molecular basis for an inherited pain syndrome. Trends Mol Med 11:555–62. PMID:16278094. doi:10.1016/j.molmed.2005.10.004
Fertleman CR, Baker MD, Parker KA, Moffatt S, Elmslie FV, Abrahamsen B, Ostman J, Klugbauer N, Wood JN, Gardiner RM, Rees M (2006) SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron 52:767–74. PMID:17145499. doi:10.1016/j.neuron.2006.10.006
Drenth JP, Waxman SG (2007) Mutations in sodium-channel gene SCN9A cause a spectrum of human genetic pain disorders. J Clin Invest. 117:3603–3609
Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, Al-Gazali L, Hamamy H, Valente EM, Gorman S, Williams R, McHale DP, Wood JN, Gribble FM, Woods CG (2006) An SCN9A channelopathy causes congenital inability to experience pain. Nature 444:894–8. PMID:17167479. doi:10.1038/nature05413
Reimann F, Cox JJ, Belfer I, Diatchenko L, Zaykin DV, McHale DP, Drenth JP, Dai F, Wheeler J, Sanders F, Wood L, Wu TX, Karppinen J, Nikolajsen L, Männikkö M, Max MB, Kiselycznyk C, Poddar M, Te Morsche RH, Smith S, Gibson D, Kelempisioti A, Maixner W, Gribble FM, Woods CG (2010) Pain perception is altered by a nucleotide polymorphism in SCN9A. Proc Natl Acad Sci USA 107:5148–5153
Valdes AM, Arden NK, Vaughn FL, Doherty SA, Leaverton PE, Zhang W, Muir KR, Rampersaud E, Dennison EM, Edwards MH, Jameson KA, Javaid MK, Spector TD, Cooper C, Maciewicz RA, Doherty M (2011) Role of the Nav1.7 R1150 W amino acid change in susceptibility to symptomatic knee osteoarthritis and multiple regional pain. Arthritis Care Res (Hoboken):440–4
Black JA, Nikolajsen L, Kroner K, Jensen TS, Waxman SG (2008) Multiple sodium channel isoforms and mitogen-activated protein kinases are present in painful human neuromas. Ann Neurol. 64:644–653
Takasaki I, Andoh T, Shiraki K, Kuraishi Y (2000) Allodynia and hyperalgesia induced by herpes simplex virus type-1 infection in mice. Pain 86:95–101
Ohtori S, Takahashi K, Aoki Y, Doya H, Ozawa T, Saito T, Moriya H (2004) Spinal neural cyclooxygenase-2 mediates pain caused in a rat model of lumbar disk herniation. J Pain. 5:385–391
Murata Y, Onda A, Rydevik B, Takahashi K, Olmarker K (2004) Distribution and appearance of tumor necrosis factor-alpha in the dorsal root ganglion exposed to experimental disc herniation in rats. Spine 29:2235–2241
Obata K, Tsujino H, Yamanaka H, Yi D, Fukuoka T, Hashimoto N, Yonenobu K, Yoshikawa H, Noguchi K (2002) Expression of neurotrophic factors in the dorsal root ganglion in a rat model of lumbar disc herniation. Pain 99:121–132
Onda A, Murata Y, Rydevik B, Larsson K, Kikuchi S, Olmarker K (2004) Infliximab attenuates immunoreactivity of brain-derived neurotrophic factor in a rat model of herniated nucleus pulposus. Spine 29:1857–1861
Olmarker K, Rydevik B (2001) Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve conduction velocity: possible implications for future pharmacologic treatment strategies of sciatica. Spine 26:863–869
Sakuma T, Kamoda H, Miyagi M, Ishikawa T, Arai G, Eguchi Y, Suzuki M, Oikawa Y, Sakuma Y, Kubota G, Inage K, Saino T, Orita S, Yamauchi K, Inoue G, Takahashi K, Ohtori S (2013) Comparison of CatWalk analysis and von Frey testing for pain assessment in a rat model of nerve crush plus inflammation. Spine (Phila Pa 1976) 38(15):E919–E924 (Epub ahead of print)
Norimoto M, Ohtori S, Yamashita M, Inoue G, Yamauchi K, Koshi T, Suzuki M, Orita S, Eguchi Y, Sugiura A, Ochiai N, Takaso M, Takahashi K (2008) Direct application of the TNF-alpha inhibitor, etanercept, does not affect CGRP expression and phenotypic change of DRG neurons following application of nucleus pulposus onto injured sciatic nerves in rats. Spine (Phila Pa 1976) 33(22):2403–2408
Yamashita M, Ohtori S, Koshi T, Inoue G, Yamauchi K, Suzuki M, Takahashi K (2008) Tumor necrosis factor-alpha in the nucleus pulposus mediates radicular pain, but not increase of inflammatory peptide, associated with nerve damage in mice. Spine (Phila Pa 1976) 33(17):1836–1842
Coggeshall RE, Tate S, Carlton SM (2004) Differential expression of tetrodotoxin-resistant sodium channels Nav1.8 and Nav1.9 in normal and inflamed rats. Neurosci Lett 355:45–48
Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG (2004) Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain 108:237–247
Strickland IT, Martindale JC, Woodhams PL, Reeve AJ, Chessell IP, McQueen DS (2008) Changes in the expression of NaV1.7, NaV1.8 and NaV1.9 in a distinct population of dorsal root ganglia innervating the rat knee joint in a model of chronic inflammatory joint pain. Eur J Pain 12:564–572
Nassar MA, Stirling LC, Forlani G, Baker MD, Matthews EA, Dickenson AH, Wood JN (2004) Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci USA 101:12706–12711
Yeomans DC, Levinson SR, Peters MC, Koszowski AG, Tzabazis AZ, Gilly WF, Wilson SP (2005) Decrease in inflammatory hyperalgesia by herpes vector-mediated knockdown of Nav1.7 sodium channels in primary afferents. Hum Gene Ther 16:271–277
Shields SD, Cheng X, Uçeyler N, Sommer C, Dib-Hajj SD, Waxman SG (2012) Sodium channel Na(v)1.7 is essential for lowering heat pain threshold after burn injury. J Neurosci 10819–10832
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mukai, M., Sakuma, Y., Suzuki, M. et al. Evaluation of behavior and expression of NaV1.7 in dorsal root ganglia after sciatic nerve compression and application of nucleus pulposus in rats. Eur Spine J 23, 463–468 (2014). https://doi.org/10.1007/s00586-013-3076-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-013-3076-y