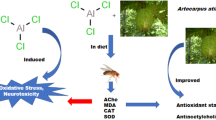
Abstract
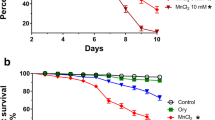
Aluminum (Al) toxicity has been associated with multiple neurodegenerative disorders, including Alzheimer’s disease (AD). Since current medications for AD and Al-related toxicity are reported to have significant side effects, the search for therapeutic agents possessing antioxidant and anticholinesterase potential is ongoing. One such agent is rutin, a flavonoid, reported for its potent antioxidant and anti-inflammatory effects. This study investigated the activity of rutin in mitigating Al chloride (AlCl3) toxicity in Drosophila melanogaster. Flies were divided into six groups containing fifty (50) flies each. Group A served as the control; Group B received 40 mM AlCl3; Groups C and D were co-treated with 40 mM AlCl3 + 0.5 mg/kg rutin and 40 mM AlCl3 + 1 mg/kg, respectively; Groups E and F were treated with rutin alone in doses of 0.5 mg/kg and 1 mg/kg, respectively, all through their diet. Negative geotaxis, superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA), and acetylcholinesterase (AChE) activities were evaluated at the end of the study. A marked decrease (P < 0.05) was noticed in survival rate, negative geotaxis, and SOD as well as a significant increase in MDA and AChE in the AlCl3-exposed flies when compared to the control. Conversely, in the groups co-treated with rutin, there was a significant attenuation of the negative effects of AlCl3. Taken together, these findings suggest that rutin protected against AlCl3, thus indicating the possible therapeutic effects of rutin against aluminum toxicity and its related disorders.







Similar content being viewed by others
References
Abolaji AO, Kamdem JP, Farombi EO, Rocha JBT (2013) Drosophila melanogaster as a promising model organism in toxicological studies. Arch Basic Appl Med 1:33–38
Abolaji AO, Olaiya CO, Oluwadahunsi OJ, Farombi EO (2017) Dietary consumption of monosodium L-glutamate induces adaptive response and reduction in the life span of Drosophila melanogaster. Cell Biochem Funct 35(3):164–170
Abolaji AO, Adedara AO, Adie MA, Vicente-Crespo M, Farombi EO (2018) Resveratrol prolongs lifespan and improves 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced oxidative damage and behavioural deficits in Drosophila melanogaster. Biochem Biophys Res Commun 503(2):1042–1048
Adedayo BC, Ogunsuyi OB, Akinniyi ST, Oboh G (2022) Effect of Andrographis paniculata and Phyllanthus amarus leaf extracts on selected biochemical indices in Drosophila melanogaster model of neurotoxicity. Drug Chem Toxicol 45(1):407–416
Ahmad Rather M, Justin-Thenmozhi A, Manivasagam T, Saravanababu C, Guillemin GJ, Essa MM (2019) Asiatic acid attenuated aluminum chloride-induced tau pathology, oxidative stress and apoptosis via AKT/GSK-3β signaling pathway in Wistar rats. Neurotox Res 35:955–968
Akinsanmi AO, Balogun O, Titlayo JO, Longdet IY, John AC (2019) The pro-oxidant effect of dextran sodium sulphate on oxidative stress biomarkers and antioxidant enzymes in Drososphila melanogaster. Afr J Biochem Res 13(6):82–89
Akpanyung EO, Bassey UE, Noah UT, Effiong GS (2020) Effects of ethanol leaf extract of Vernonia amygdalina on some indices of liver function, oxidative stress and lipid profile in aluminium chloride intoxicated male Wistar rats. Biokemistri 32(1):11–21
Alasfar RH, Isaifan RJ (2021) Aluminum environmental pollution: the silent killer. Environ Sci Pollut Res 28(33):44587–44597
Arowoogun J, Akanni OO, Adefisan AO, Owumi SE, Tijani AS, Adaramoye OA (2021) Rutin ameliorates copper sulfate-induced brain damage via antioxidative and anti-inflammatory activities in rats. J Biochem Mol Toxicol 35(1):e22623
Beyer W, Imlay J, Fridovich I (1991) Superoxide dismutases. Prog Nucleic Acid Res Mol Biol 40:221–253
Cheeseman KH (1993) Mechanisms and effects of lipid peroxidation. Mol Aspects Med 14(3):191–197
Cohen G, Dembiec D, Marcus J (1970) Measurement of catalase activity in tissue extracts. Anal Biochem 34(1):30–38
Colović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM (2013) Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol 11(3):315–335
Dave KR, Syal AR, Katyare SS (2002) Effect of long-term aluminum feeding on kinetics attributes of tissue cholinesterases. Brain Res Bull 58(2):225–233
Efosa JO, Omage K, Azeke MA (2023) Hibiscus sabdariffa calyx protect against oxidative stress and aluminium chloride-induced neurotoxicity in the brain of experimental rats. Toxicol Rep 10:469–480
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95
Enogieru AB, Haylett W, Hiss DC, Bardien S, Ekpo OE (2018) Rutin as a potent antioxidant: implications for neurodegenerative disorders. Oxid Med Cell Longev 2018
Farombi EO, Abolaji AO, Farombi TH, Oropo AS, Owoje OA, Awunah MT (2018) Garcinia kola seed biflavonoid fraction (Kolaviron), increases longevity and attenuates rotenone-induced toxicity in Drosophila melanogaster. Pestic Biochem Physiol 145:39–45
Fidelis KR, dos Santos Nunes RG, da Silva CS, Oliveira CVB, Costa AR, de Lima Silva JR, ... Barros LM (2021) Evaluation of the neuroprotective effect of rutin on Drosophila melanogaster about behavioral and biochemical aspects induced by mercury chloride (HgCl2). Comp Biochem Physiol Part C Toxicol Pharmacol 249:109119
Gargano JW, Martin I, Bhandari P, Grotewiel MS (2005) Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp Gerontol 40(5):386–395
Gaweł S, Wardas M, Niedworok E, Wardas P (2004) Malondialdehyde (MDA) as a lipid peroxidation marker. Wiadomosci lekarskie (Warsaw, Poland: 1960) 57(9–10):453–455
Igbokwe IO, Igwenagu E, Igbokwe NA (2019) Aluminium toxicosis: a review of toxic actions and effects. Interdiscip Toxicol 12(2):45
Igharo OG, Abadaike LI, Osunbor JO, Owie IC, Aruomaren AI, Igharo EL, Ikeke KI (2021) Effect of dietary inclusion of chitosan on survival and selected cellular antioxidants in Drosophila melanogaster. J Appl Sci Environ Manag 25(6):1093–1098
Inneh CA, Eiya BO (2023) Anticholinesterase activity and antioxidant effect of vitamin E in aluminium chloride induced toxicity in Drosophila melanogaster. J Afr Assoc Physiol Sci 11(1):17–28
Inneh C, Enogieru A (2023) Anticholinesterase and antioxidant potential of aqueous leave extract of Phyllantus amarus in aluminium chloride-induced Alzheimer’s disease in Drosophila melanogaster. Physiology 38(S1):5685820
Iova GM, Calniceanu H, Popa A, Szuhanek CA, Marcu O, Ciavoi G, Scrobota I (2021) The antioxidant effect of curcumin and rutin on oxidative stress biomarkers in experimentally induced periodontitis in hyperglycemic Wistar rats. Molecules 26(5):1332
Islam MN, Rauf A, Fahad FI, Emran TB, Mitra S, Olatunde A, ... Mubarak MS (2022) Superoxide dismutase: an updated review on its health benefits and industrial applications. Crit Rev Food Sci Nutr 62(26):7282–7300
Jennings BH (2011) Drosophila–a versatile model in biology medicine. Mater Today 14(5):190–195
Klein GL (2019) Aluminum toxicity to bone: a multisystem effect? Osteoporosis Sarcopenia 5(1):2–5
Korkmaz A, Kolankaya D (2010) Protective effect of rutin on the ischemia/reperfusion induced damage in rat kidney. J Surg Res 164(2):309–315
Kumar A, Dogra S, Prakash A (2009) Protective effect of curcumin (Curcuma longa), against aluminium toxicity: possible behavioral and biochemical alterations in rats. Behav Brain Res 205(2):384–390
Liaquat L, Sadir S, Batool Z, Tabassum S, Shahzad S, Afzal A, Haider S (2019) Acute aluminum chloride toxicity revisited: study on DNA damage and histopathological, biochemical and neurochemical alterations in rat brain. Life Sci 217:202–211
McGurk L, Berson A, Bonini NM (2015) Drosophila as an in vivo model for human neurodegenerative disease. Genetics 201(2):377–402
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247(10):3170–3175
Oboh G, Adebayo AA, Ademosun AO, Olowokere OG (2020) Rutin restores neurobehavioral deficits via alterations in cadmium bioavailability in the brain of rats exposed to cadmium. Neurotoxicol 77:12–19
Oboh G, Oladun FL, Ademosun AO, Ogunsuyi OB (2021) Anticholinesterase activity and antioxidant properties of Heinsia crinita and Pterocarpus soyauxii in Drosophila melanogaster model. J Ayurveda Integr Med 12(2):254–260
Oyetayo BO, Abolaji AO, Fasae KD, Aderibigbe A (2020) Ameliorative role of diets fortified with curcumin in a Drosophila melanogaster model of aluminum chloride-induced neurotoxicity. J Funct Foods 71:104035
Prasad R, Prasad SB (2019) A review on the chemistry and biological properties of rutin, a promising nutraceutical agent. Asian J Pharm Pharmacology 5(S1):1–20
Protchenko O, Baratz E, Jadhav S, Li F, Shakoury-Elizeh M, Gavrilova O, ... Philpott CC (2021) Iron chaperone poly rC binding protein 1 protects mouse liver from lipid peroxidation and steatosis. Hepatol 73(3):1176–1193
Qu S, Dai C, Guo H, Wang C, Hao Z, Tang Q, ... Zhang Y (2019) Rutin attenuates vancomycin-induced renal tubular cell apoptosis via suppression of apoptosis, mitochondrial dysfunction, and oxidative stress. Phytother Res 33(8):2056–2063
Rhondenizer D, Martin I, Bhandari P, Pletcher SD, Grotewiel M (2008) Genetic and environmental factors impact age-related impairment of negative geotaxis in Drosophila by altering age-dependent climbing speed. Exp Gerontol 43(8):739–748
Rotimi DE, Elebiyo TC, Ojo OA (2023) Therapeutic potential of rutin in male infertility: a mini review. J Integr Med
Shangari N, O’Brien PJ (2006) Catalase activity assays. Curr Protoc Toxicol 27(1):7–7
Taqui R, Debnath M, Ahmed S, Ghosh A (2022) Advances on plant extracts and phytocompounds with acetylcholinesterase inhibition activity for possible treatment of Alzheimer’s disease. Phytomedicine Plus 2(1):100184
Uthra C, Reshi MS, Jaswal A, Yadav D, Shrivastava S, Sinha N, Shukla S (2022) Protective efficacy of rutin against acrylamide-induced oxidative stress, biochemical alterations and histopathological lesions in rats. Toxicol Res 11(1):215–225
Varshney R, Kale RK (1990) Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Radiat Biol 58(5):733–743
Wang F, Liu M, Niu X, Xia L, Qu F (2023) Dextran-assisted ultrasonic exfoliation of two-dimensional metal-organic frameworks to evaluate acetylcholinesterase activity and inhibitor screening. Anal Chim Acta 1243:340815
Xie Y, Yang W, Chen X, Xiao J (2014) Inhibition of flavonoids on acetylcholine esterase: binding and structure–activity relationship. Food Funct 5(10):2582–2589
Zatta P, Ibn-Lkhayat-Idrissi M, Zambenedetti P, Kilyen M, Kiss T (2002) In vivo and in vitro effects of aluminum on the activity of mouse brain acetylcholinesterase. Brain Res Bull 59(1):41–45
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was not supported by any funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Research Ethics Committee, College of Medical Sciences, University of Benin, with approval no. CMS/REC/2023/356.
Informed consent
For this type of study, informed consent is not required.
Consent for publication
For this type of study, consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iyiegbu, M.E., Enogieru, A.B. Antioxidant and anticholinesterase activity of rutin in aluminum chloride-exposed Drosophila melanogaster. Comp Clin Pathol (2024). https://doi.org/10.1007/s00580-024-03564-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00580-024-03564-8