Abstract
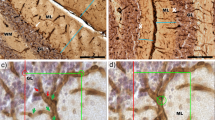
Dystrophic or swollen axons occur in a variety of neurological disorders. Although the structures are often viewed as an incidental or age-related phenomenon, there are few reports that actually described axonal and dendritic swellings on cerebellar Purkinje cells in domestic animals with no apparent neurological clinical signs. A 12-year-old Holstein-Friesian dairy cow, which exhibited no apparent cerebellar or other significant clinical signs, was culled from old age. On histopathology of the cerebellum, there were Purkinje cell axonal torpedoes and axonal spheroids involving nerve fibers in the folial and medullary white matter, which were identified by Bodian’s silver impregnation technique and anti-neuron-specific enolase (NSE) immunohistochemical stain. Less frequently, dendritic swellings on Purkinje cells were found in the molecular layer. Cell bodies of Purkinje cells appeared otherwise normal and were not accompanied by Bergmann glia proliferation. Immunohistochemistry for glial fibrillary acid protein (GFAP) failed to demonstrate significant fibrillary gliosis. Deposition of lipofuscin pigments was seen in the cortical and medullary neurons and glial cells. Any sites of the cerebellum showed no evidence of neuronal cell degeneration, glial reaction, cellular infiltrates, or vascular lesions. Morphometrically, there was no significant decrease in number of Purkinje cells in comparison with younger control cows (n = 2). Purkinje cell axonal torpedoes or axonal spheroids were absent in the cerebellum of younger control cows (n = 10). The precise mechanism for the development of swollen Purkinje cell axons and dendrites remains to be determined, although an association with advanced age was considered one of the likeliest possibilities.







Similar content being viewed by others
References
Babij R, Lee M, Cortes E et al (2013) Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain 136:3051–3061
Bäurle J, Grässer-Cornehls U (1994) Axonal torpedoes in cerebellar Purkinje cells of two normal mouse strains during aging. Acta Neuropathol 88:237–245
Beech J (1984) Neuroaxonal dystrophy of the accessory cuneate nucleus in horses. Vet Pathol 21:384–393
Borras D, Ferrer I, Pumarola M (1999) Age-related changes in the brain of the dog. Vet Pathol 36:202–211
Cavanagh JB, Gysbers MF (1983) Ultrastructural features of the Purkinje cell damage caused by acrylamide in the rat: a new phenomenon in cellular neuropathology. J Neurocytol 12:413–437
de Lahunta A (1983) Veterinary neuroanatomy and clinical neurology. WB Saunders, Philadelphia, pp 255–278
de Lahunta A (1990) Abiotrophy in domestic animals. Can J Vet Res 54:65–76
Ferrer I, Cusi V, Pineda M et al (1988) Focal dendritic swellings in Purkinje cells in mucopolysaccharidoses types I, II and III. A Golgi and ultrastructural study. Neuropathol Appl Neurobiol 14:315–323
Friede RL (1989) Developmental neuropathology. Springer-Verlag, Berlin, pp 552–560
Fyfe JC, Al-Tamimi RA, Liu J et al (2011) A novel mitofusin 2 mutation causes canine fetal-onset neuroaxonal dystrophy. Neurogenetics 12:223–232
Ganjali H, Ganjali M (2013) Fixation in tissue processing. Intl J Farm and Alli Sci 2:686–689
Gravel C, Leclerc N, Plioplys A et al (1986) Focal axonal swellings in rat cerebellar Purkinje cells during normal development. Brain Res 363:325–332
Gavier-Widen D, Wells GAH, Simmons MM et al (2001) Histological observations on the brains of symptomless 7-year-old cattle. J Comp Pathol 124:52–59
Grimaldi G, Manto M (2012) Topography of cerebellar deficits in humans. Cerebellum 11:336–351
Hagan CE, Bolon B, Keene D (2012) Nervous system. In: Treuting PM, Dintzis SM (eds) Comparative anatomy and histology: a mouse and human atlas. Elsevier, Amsterdam, pp 339–394
Hanshaw DM, Finnie JW, Manavis J et al (2015) Axonal spheroid accumulation in the brainstem and spinal cord of a young Angus cow with ataxia. Aust Vet J 93:283–286
Hopwood D (1996) Fixation and fixatives. In: Bancroft J, Stevens A (eds) Theory and practice of histological techniques. Churchill Livingstone, New York, pp 23–45
Horoupian DS (1982) ‘Dystrophic’ Purkinje cell dendrites in an infant. Acta Neuropathol 57:165–170
Ince PG, Clark B, Holton J et al (2008) Disorders of movement and system degenerations. In: Love S, Louis DN, Ellison DW (eds) Greenfield’s neuropathology, 8th edn. Hodder Arnold, London, pp 889–1030
Jahns H, Callanan JJ, McElroy MC et al (2006) Age-related and non-age-related changes in 100 surveyed horse brains. Vet Pathol 43:740–750
Jeffrey M (1992) A neuropathological survey of brains submitted under the Bovine Spongiform Encephalopathy Orders in Scotland. Vet Rec 131:332–337
Jubb KVF, Huxtable CR (1993) The nervous system. In: Jubb KVF, Kennedy PC, Palmer N (eds) Pathology of domestic animals, 4th edn. Academic Press, San Diego, pp 267–439
Kageyama K (1973) Manual of histologic techniques, 4th edn. Igaku Shoin, Tokyo (in Japanese)
Kato T, Hirano A (1985) A Golgi study of proximal portion of the human Purkinje cell axon. Acta Neuropathol 68:191–195
Kemp KC, Cook AJ, Redondo J et al (2016) Purkinje cell injury, structural plasticity and fusion in patients with Friedreich’s ataxia. Acta Neuropathol Comm 4:67
Kessell AE, Finnie JW, Blumbergs PC et al (2012) Neuroaxonal dystrophy in Australian Merino lambs. J Comp Pathol 147:62–72
Ljungberg L, Lang-Ouellette D, Yang A et al (2016) Transient developmental Purkinje cell axonal torpedoes in healthy and ataxic mouse cerebellum. Front Cell Neurosci 10:248
Louis ED, Faust PL, Ma KJ et al (2011) Torpedoes in the cerebellar vermis in essential tremor cases vs. controls. Cerebellum 10:812–819
Louis ED, Faust PL, Vonsattel JPG et al (2007) Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 130:3297–3307
Louis ED, Faust PL, Vonsattel JPG et al (2009a) Purkinje cell axonal torpedoes are unrelated to advanced aging and likely reflect cerebellar injury. Acta Neuropathol 117:719–721
Louis ED, Faust PL, Vonsattel JPG et al (2009b) Torpedoes in Parkinson’s disease, Alzheimer’s disease, essential tremor, and control brains. Mov Disord 24:1600–1605
Lowe J, Mirra SS, Hyman BT (2008) Aging and dementia. In: Love S, Louis DN, Ellison DW (eds) Greenfield’s neuropathology, 8th ed. Hodder Arnold, London, pp. 1031-1152
Moeller JJ, Macaulay RJ, Valdmanis PN et al (2008) Autosomal dominant sensory ataxia: a neuroaxonal dystrophy. Acta Neuropathol 116:331–336
Redondo J, Kemp K, Hares K et al (2015) Purkinje cell pathology and loss in multiple sclerosis cerebellum. Brain Pathol 25:692–700
Resibois A, Poncelet L (2004) Purkinje cell neuroaxonal dystrophy similar to nervous mutant mice in two sibling kittens. Acta Neuropathol 107:553–558
Rodriquez-Costa T, Cabello A, Recuero-Fernandez E et al (2001) Infantile neuroaxonal dystrophy. A report of two cases and a review of the literature published over the past ten years. Rev Neurol 33:443–447
Saito K (1980) Spheroids and altered axons in the spinal gray matter of the normal cat. An electron-microscopic study. Acta neuropathol 52:213–222
Sawada K, Fukui Y (2011) Development of Purkinje cell axonal torpedoes in the cerebellum of rolling mouse Nagoya. Curr Neurobiol 2:43–47
Schindler D, Bishop DF, Wolfe DE et al (1989) Neuroaxonal dystrophy due to lysosomal alpha-N- acetylgalactosaminidase deficiency. N Engl J Med 320:1735–1740
Seitelberger F (1986) Neuroaxonal dustrophy: its relation to aging and neurological disease. In: Vinken PJ, Bruyn GW, Klawans HL (eds) Handbook of clinical neurology. Elsevier Science, Amsterdam, pp 391–495
Seno Y (1989) Morphomeric analysis of the axonal torpedo of Purkinje cells—relationship with aging and disease. Kitakanto Med J 39:465–474
Slayter MV, Summers BA, Meade RP et al (1998) Axonal spheroids in the cochlear nucleus of normal beagle dogs. Vet Pathol 35:150–153
Summers BA, Cummings JF, de Lahunta A (1995) Veterinary neuropathology. Mosby-Year Book, Saint Louis, pp 49–67
Takahashi N, Iwatsubo T, Nakano I et al (1992) Focal appearance of cerebellar torpedoes associated with discrete lesions in the cerebellar white matter. Acta Neuropathol 84:153–156
Valberg SJ, Lewis SS, Shivers JL et al (2015) The equine movement disorder “shivers” is associated with selective cerebellar Purkinje cell axonal degeneration. Vet Pathol 52:1087–1098
Youssef SA, Capuccino MT, Rofina JE et al (2016) Pathology of aging brain in domestic and laboratory animals, and animal models of human neurodegenerative diseases. Vet Pathol 53:327–348
Yu M, Ma K, Faust PL et al (2012) Increased number of Purkinje cell dendritic swellings in essential tremor. Eur J Neurol 19:625–630
Funding
This study was not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Ohfuji, S. Axonal and dendritic swellings on cerebellar Purkinje cells in a cow: a possible age-related change. Comp Clin Pathol 26, 1381–1387 (2017). https://doi.org/10.1007/s00580-017-2544-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-017-2544-x