Abstract
Arbuscular mycorrhizal fungi (AMF) can increase plant tolerance and/or resistance to pests such as the root-knot nematode Meloidogyne incognita. However, the ameliorative effects may depend on AMF species. The aim of this work was therefore to evaluate whether four AMF species differentially affect plant performance in response to M. incognita infection. Tomato plants grown in greenhouse conditions were inoculated with four different AMF isolates (Claroideoglomus claroideum, Funneliformis mosseae, Gigaspora margarita, and Rhizophagus intraradices) and infected with 100 second stage juveniles of M. incognita at two different times: simultaneously or 2 weeks after the inoculation with AMF. After 60 days, the number of galls, egg masses, and reproduction factor of the nematodes were assessed along with plant biomass, phosphorus (P), and nitrogen concentrations in roots and shoots and root colonization by AMF. Only the simultaneous nematode inoculation without AMF caused a large reduction in plant shoot biomass, while all AMF species were able to ameliorate this effect and improve plant P uptake. The AMF isolates responded differently to the interaction with nematodes, either increasing the frequency of vesicles (C. claroideum) or reducing the number of arbuscules (F. mosseae and Gi. margarita). AMF inoculation did not decrease galls; however, it reduced the number of egg masses per gall in nematode simultaneous inoculation, except for C. claroideum. This work shows the importance of biotic stress alleviation associated with an improvement in P uptake and mediated by four different AMF species, irrespective of their fungal root colonization levels and specific interactions with the parasite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food production is a major concern for the growing human population. The intensive use of soil to increase its productivity has led to its degradation, affecting ecosystems, environment, and human health. Consequently, there is a need to seek sustainable solutions and new approaches in agriculture to restore the quality of ecosystem services, in which soil microbiota are crucial. Among soil microorganisms, arbuscular mycorrhizal fungi (AMF) establish mutualistic relationships by colonizing the roots of most plant species (Turrini et al. 2018). In addition to nutritional benefits, AMF can increase plant tolerance and/or resistance to abiotic and biotic stresses such as pathogenic fungi, bacteria, viruses, parasitic plants, and nematodes (Diagne et al. 2020). Mycorrhizal protection against biotic stress is becoming widely acknowledged in the context of plant production and is subject to the influences of various biotic and abiotic factors (Dowarah et al. 2022). In this sense, different AMF species differ in their interactions with host plants, yet the mechanisms behind these interaction differences require further investigation (Marro et al. 2022).
Plant-parasitic nematodes (PPN) establish an intimate relationship with their host in which they can reprogram plant cells for their own benefit (Phani et al. 2021). There are more than 4100 species of PPN, and some of them have great impact on agriculture and horticulture worldwide, accounting for annual losses of £173 billion (Decraemer and Hunt 2006). Among PPN, Meloidogyne (Tylenchida - Phylum: Nematoda), commonly known as the root-knot nematode, is one of the most devastating genera (Jones et al. 2013). The second stage juvenile (J2) penetrates the roots through a stylet and migrates close to the vascular cylinder, where it selects several cells to induce the formation of a feeding site (Perry and Moens 2011) and become sedentary. The surrounding cortex cells divide and become hypertrophied and the pericycle cells proliferate resulting in the formation of a root swelling known as a gall. This alters the root’s uptake of nutrients and causes symptoms of wilting and yield reduction (Castagnone-Sereno et al. 2013). Inside the gall, the J2 moults to J3, J4 and finally adult female, acquiring a pear-shaped form. When mature, the female lays eggs in an egg mass consisting of a gelatinous matrix on the root surface. The first juvenile molts within the egg to the infective or J2 stage, which emerges from the egg into the soil and can seek new hosts (Subedi et al. 2020). So far, the main management strategy used against this pest is through chemical control, which has detrimental effects on soils and non-target species (Abd-Elgawad 2021). Resistant genes against Meloidogyne spp. have also been described for tomato (Mi genes) (e.g., Ammiraju et al. 2003) and some other horticultural species (see Escobar et al. 2015). However, resistant cultivars are not effective against certain breeds of Meloidogyne spp. and can lose their resistance at high soil temperatures (de Almeida et al. 2020). The use of AMF as a biological control agent against PPN has been documented in the scientific literature, as AMF can reduce PPN populations (e.g., Castillo et al. 2006; Herrera-Parra et al. 2021; Marro et al. 2014, 2018). Proposed mechanisms of PPN control include direct effects, such as competition for space and nutrients, and indirect (plant-mediated) effects (Schouteden et al. 2015). AMF could protect roots from nematode invasion, either through their intricate hyphal networks in the soil or through competition for space in the roots. However, this hypothesis has yet to be proven (Dowarah et al. 2022). With regards to plant-mediated responses to nematodes, mycorrhizal plants might be more capable of handling the stress caused by Meloidogyne spp. because of enhanced ability to recover from damage following root nematode infection (Banuelos et al. 2014). Moreover, AMF may induce either localized and systemic resistance mechanisms in plants (Vos et al. 2012b) by inducing a priming effect (Vos et al. 2013; Molinari and Leonetti 2019; Pozo and Azcón-Aguilar 2007) and can reduce root penetration by PPN through changes in root exudates (Vos et al. 2012a, b).
The biocontrol effect of AMF against PPN depends on several factors. One of them may be the AMF species involved, as different AMF isolates are expected to affect plant growth, nutrition, and stress responses differently (Mensah et al. 2015; Munkvold et al. 2004; Sikes et al. 2009). Most studies testing plant responses to AMF have used Rhizophagus irregularis and Funneliformis mosseae (Glomerales), as they are considered generalists and ubiquitous species; however, these species can be quite heterogeneous in their functionality (Berruti et al. 2016). It has been observed that either Rhizophagus spp. or F. mosseae have an advantage over other AMF species in root colonization on different plant hosts (e.g., Blažková et al. 2021; Carrara and Heller 2022; Jansa et al. 2008; Säle et al. 2021; Voříšková et al. 2019). A rapid colonization rate could provide them with greater ability to compete with PPN for space in the rhizosphere and/or the roots. Regarding nutritional effects, it has been demonstrated, for example, that plants inoculated with F. mosseae have highly efficient water uptake (Marulanda et al. 2003), while those with Rhizophagus spp. have enhanced P acquisition, and plants with Claroideoglomus spp. and Gigaspora spp. show increased magnesium and calcium uptake (Carrara and Heller 2022). Also, a recent study found that different genetic organization (dikaryotic versus homokaryotic) of strains within the same species (Rhizophagus irregularis) can differentially affect the response of plants to mycorrhizas (Terry et al. 2023). Such differences may contribute to different tolerance of the host plant to nematode attack.
Finally, AMF species induce different systemic responses in plants, which may lead to differences in resistance to nematodes. Immunity in plants is regulated by several phytohormones, mainly salicylic acid (SA), jasmonic acid (JA), and ethylene (ET). During AMF colonization of roots, plant defenses are activated and a cross-talk between SA and JA occurs. Mycorrhizal symbiosis primes the plant tissues for rapid effective activation of JA-dependent defenses upon attack, resulting in enhanced resistance (Pozo and Azcón-Aguilar 2007). This priming effect has been observed in tomato plants with a mixture of AMF, antagonistic fungi, and rhizobacteria against M. incognita by the up-regulation of various tested genes, such as PR- and ACO (Molinari and Leonetti 2019). Moreover, a study comparing the transcriptional response of tomato to F. mosseae and R. irregularis found that, although both species induced common oxylipin pathway genes related to JA biosynthesis, the overall transcriptional profiles by these two AMF were different (López-Ráez et al. 2010). Interestingly, the oxylipin pathway has been shown to be involved in plant nematode responses (Gao et al. 2008).
Although several studies have investigated the interactions among different plant species, nematodes, and AMF, they generally focus more on biocontrol than on nutrient acquisition. The novelty of this study lies in simultaneously comparing AMF species belonging to different families to explore whether they differentially affect plant nutrition and responses to nematode infection. We selected tomato (Solanum lycopersicum L.), a globally cultivated crop susceptible to several pests and pathogens including M. incognita, to evaluate whether four AMF species of contrasting functional characteristics differentially affect (a) plant stress responses to M. incognita infection and (b) development and reproduction of the nematode in relation with the plant response to AMF inoculation.
Materials and methods
Experimental design
The experimental design combined two factors in a full factorial manner. The first factor was inoculation with AMF, comprising five levels: uninoculated control (NM); and inoculation with one of four isolates: (1) Rhizophagus intraradices C. Walker and Schuessler (2010), isolate PH5 (RI), (2) Claroideoglomus claroideum C. Walker and Schuessler (2010), isolate BEG23 (CC), (3) Gigaspora margarita W. N. Becker & I. R. Hall (1976), isolate BEG34 (GM), and (4) Funneliformis mosseae C. Walker and Schuessler (2010), isolate BEG95 (FM). The second factor was infection with the nematode Meloidogyne incognita Kofoid and White (1919), with three levels: no nematodes added (NN), nematodes inoculated simultaneously with AMF at the establishment of the experiment (NS), and nematodes inoculated 14 days after the AMF inoculation (NP). Two different times of nematode application were included because the timing of Meloidogyne infection in relation to transplanting time and mycorrhiza establishment may play a role in the interaction (Talavera et al. 2001). Each treatment had 5 independent replicates (5 × 3 × 5 = 75 plants in total). The experiment was harvested 60 days after its establishment.
Plant material and substrate
Tomato seeds (cv. Money maker) were surface sterilized in 10% NaOCl for 5 min, washed with distilled water, and sown in Petri dishes for germination at room temperature. Seedlings were grown in trays in a mixture of sterile sand and zeolite (1:1) for 4 weeks. The experiment was established by transplanting four-leafed seedlings individually into experimental pots (11 cm height × 13 cm diameter) containing 700 ml of the same substrate as used for pre-cultivation.
Inoculation with AMF and nematodes
The AMF isolates are maintained at the Department of Mycorrhizal Symbiosis (Institute of Botany, Czech Academy of Sciences, Průhonice, Czech Republic) in an inert sand-zeolite mixture (1:1, v: v) with Desmodium sp. as host plant. The inoculum of each isolate consisted of homogenized substrate of approximately 6-month-old cultures with chopped roots, air-dried for 1 day. At the time of transplanting, each seedling was inoculated with 30 ml of the treatment-specific AMF inoculum mixed with 270 ml of sterile sand-zeolite (1:1, v: v) as a central substrate layer in the pot. For Gi. margarita only, 100 spores per pot, collected from sporulating cultures, were used as inoculum, as spores are the main infective propagules of Gigaspora spp. (Klironomos and Hart 2002). The control plants (NM) received the same amount of heat-sterilized substrate of AMF inoculum (autoclaved twice at 121 °C for 30 min, 24 h apart). To include similar soil microorganisms other than AMF into the NM treatments, 5 ml of a microbial filtrate was added to each pot: this was prepared by shaking 100 g of the nonsterile soil from a culture substrate with 1 L of deionized water for 30 min and filtering twice through filter paper (pore size 10 μm).
An isolate of M. incognita race 1 was obtained from roots of naturally infected Vitis rootstocks (Richter 110) at Bollullos par del Condado (Huelva province, Spain) (Gutiérrez-Gutiérrez et al. 2011). To establish and maintain M. incognita, the nematode isolate was raised on tomato plants starting from a single egg mass and subsequently reared in pots containing a mixture of sterile sand and zeolite (1:1) in which tomato plants (cv. Money maker) were grown under greenhouse conditions. After approximately 2 months, egg masses were extracted from the root galls and placed in Petri dishes containing sterile water. They were kept at room temperature (25 °C) until the eggs hatched (about 10 days). For inoculation, mobile J2 were collected with a pipette and counted under a stereomicroscope. Inoculation was performed with 100 J2 in 1 ml of distilled water by pipetting the larvae on the surface of the pot, next to the plant. This procedure was carried out in the same way for the NS and NP treatments, i.e., immediately after the establishment of the experiment or 14 days later, respectively.
Growth conditions and harvest
Plants were grown under greenhouse conditions (June–August). They were watered daily and fertilized twice a week with Hewitt’s nutrient solution (Hewitt 1966) with phosphorus reduced to 25% of the standard concentration (H2NaPO4 0.33 mM).
Sixty days after the inoculation of AMF treatments, the plants were harvested, and the roots gently washed. A sub-sample of the root system (approximately 0.5 g fresh weight) was stored in KOH for subsequent staining with trypan blue (Koske and Gemma 1989). The frequency of AMF structures in the stained roots (30 1.5-cm root segments per sample) was estimated by the grid line intersection method (Mc Gonigle et al. 1990), 100 intersecting lines per sample were scored at 100x magnification (Olympus Bx60). Hyphae, arbuscules, and vesicles, as the main intraradical structures of AMF, were scored separately, and the percentage of hyphal presence was taken as the total percentage of root colonization, as none of the scored lines intersected arbuscules or vesicles without also intersecting a hypha. The relative abundances of arbuscules (relA%) and vesicles (relV%) were calculated as their percentage frequencies within the colonized root, i.e., relA% = A / H × 100, where A and H are the frequencies of arbuscules and hyphae, respectively, as estimated by microscopy.
The remaining roots were observed under a stereomicroscope to count the number of galls and egg masses. Egg masses were removed and immersed in a 1% NaClO solution for 4 min to dissolve the gelatinous matrix (Hussey and Barker 1973), and the eggs were counted. The reproductive factor (RF) was calculated as follows: RF = final population / initial population, where final population is the number of eggs counted at the end of the experiment and initial population is the number of inoculated J2 (100). Roots and shoots were then dried at 60 °C to estimate their dry weight.
Subsamples of homogenized shoot and root biomass were ground using a Retsch MM200 mill (Retsch GmbH, Haan, Germany) to determine the P and N concentrations in the shoots of the experimental plants. N concentrations were determined using a Flash EA 2000 elemental analyzer coupled with a Delta V Advantage isotope ratio mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). P concentrations were determined after mineralization (adding 4 ml of HNO3 and 1 ml of 30% H2O2) by the photometric method based on the reaction of phosphates with ammonium molybdate, using the reaction mixture with sulphuric acid, ascorbic acid, and tartarate antimonylo-potassium (Murphy and Riley 1962). The absorbance of the resulting blue color was measured with a UV-vis spectrophotometer UV-400 Unicam at 630 nm (Zbíral 1994). The P and N contents in shoots and roots were then calculated by multiplying the concentrations by the shoot or root dry weights. Shoot to root ratios of N and P were calculated from the elements’ concentrations.
Data analysis
All collected parameters were subjected to two-way analysis of variance (ANOVA) with AMF and nematodes as the main effects including the interaction term. The Di Rienzo, Guzmán and Casanoves (DGC) test (p ≤ 0.05) was used to compare means a posteriori (Di Rienzo et al. 2002). The DGC test is based upon clustering and yields non-overlapping groups of homogenous means. When there was a significant interaction between the main factors, pairwise differences across all the treatments were considered, whereas when the interaction was not significant, differences were assessed by DGC only for significant main factors. Pearson correlation coefficients between root and shoot nutrient concentrations, dry weights, and nematode galls were performed, and their significance levels were estimated. Data were checked for normality and homogeneity of variance prior to statistical analysis. Model diagnostics were performed examining the model’s simulated quantile scaled residuals using the DHARMA package in R. The models were assessed for over-dispersion, zero-inflation, and expected distribution of the residuals (Hartig and Lohse 2020). When analyzing root colonization data, the NM control was excluded, and the data were square-root transformed to adjust for normality and homogeneity of variance. For analysis of the relV% parameter, the FM and GM inoculated treatments were excluded because these two isolates did not form vesicles. When analyzing the nematode parameters, the control without nematodes was excluded. The N shoot to root ratio was square-root transformed to adjust for normality and homogeneity of variances. A posteriori comparisons were performed on the transformed data. All these analyses were performed using the statistical software InfoStat/Professional (Infostat 2011) and its interface with the software R (R Core Team 2011).
Results
Root colonization
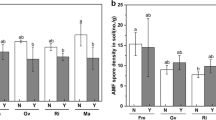
The four AMF isolates colonized roots with different rates (Table 1; Fig. 1), whereas no mycorrhization was observed in the non-inoculated plants of the NM treatment (data not shown). The highest root colonization was observed in RI plants with percentages ranging between 85 and 95%, whereas the lowest colonization rates were determined in GM (3–5%) and CC (12–21%) (Fig. 1a). No differences in root colonization were observed between the nematode treatments, nor was the interaction between the two factors significant (Table 1). The relA% was significantly influenced by the inoculation treatment (Table 1, main factor effect of AMF), lower in GM and FM than in RI and CC (Fig. 1b). Also, both nematode treatments (NS and NP) significantly reduced relA% overall (Table 1 main factor effect of Nematode). The effects of both main factors resulted in FM and GM having the lowest relA% when nematodes were applied. The relV% had a significant interaction among nematode and AMF treatments for the two fungi that formed vesicles: while nematode infection had no effect on relV% of RI, posterior nematode application increased relV% of CC (Fig. 1c, Table 1). FM and GM did not form vesicles.
a Root colonization by four arbuscular mycorrhizal fungal (AMF) isolates, b relative abundance of arbuscules (relA%), and c of vesicles (relV%) of tomato plants subjected to different M. incognita treatments: no nematodes (NN), nematodes inoculated simultaneously with the AMF at planting (NS) or 2 weeks after AMF (NP). The AMF inoculations treatments were non-inoculated control (NM), inoculated with C. claroideum (CC), F. mosseae (FM), Gi. margarita (GM), or R. intraradices (RI). Different capital letters indicate significant differences across all treatments according to the DGC test (p < 0.05). Differences according to the DGC test (p < 0.05) for the AMF main factor effect are shown as lowercase letters at the upper left of each fungus panel when the interaction with nematode inoculation is not significant. In a, data were square root transformed to adjust for normality and homogeneity of variance. The box plots show the 25% and 75% quartiles with median, 1.5 times interquartile range (as whiskers) and outliers. Outliers were included in all analyses. Black squares indicate the mean values of each treatment (n = 5). Statistics are presented in Table 1
Plant growth and nutrition
Dry weights
Root dry weight was significantly affected by nematode infection and AMF inoculation, while the interaction of both factors was non-significant (Table 2). Simultaneous infection with nematodes decreased root dry weight as compared to the other two nematode treatments (Fig. 2a), the main effect of AMF inoculation consisted in significantly lower root dry weight of CC-inoculated plants as compared to plants in the other inoculation treatments. Shoot dry weight was not affected by AMF inoculation alone, but by the interaction of the two experimental factors (Table 2), inoculation with all AMF isolates alleviated the detrimental effect of nematodes on non-mycorrhizal plants after simultaneous nematode application (Fig. 2b).
Root dry weight (a) and shoot dry weight (b) of tomato plants inoculated with four arbuscular mycorrhizal fungal (AMF) isolates and subjected to different M. incognita treatments: no nematodes (NN), nematodes inoculated simultaneously with the AMF at planting (NS) or 2 weeks after AMF (NP). The AMF inoculations treatments were non-inoculated control (NM), inoculated with C. claroideum (CC), F. mosseae (FM), Gi. margarita (GM), or R. intraradices (RI). Different capital letters indicate significant differences across all treatments according to the DGC test (p < 0.05). Differences according to the DGC test (p < 0.05) for the nematode main factor effect are shown as lowercase letters at the upper left of each fungus panel when the interaction with nematode inoculation is not significant. The box plot shows the 25% and 75% quartiles with median, 1.5 times interquartile range (as whiskers) and outliers. Outliers were included in all analyses. Black squares indicate the mean values of each treatment (n = 5). Statistics are presented in Table 2
Phosphorus and nitrogen
Inoculation with AMF alone had no effect on P and N concentrations in roots, but significantly affected them in interaction with nematode treatments (Table 2; Fig. 3a, b). For both nutrients consistently, the interaction involved increased nutrient concentrations when AMF and nematodes were applied simultaneously. The reduction in root biomass was significantly correlated with an increase in root N concentration (r= −0.3, p = 0.01) but not with an increase in root P concentration ( r= −0.2, p = 0.09) (Fig. S1a, b).
Concentrations of phosphorus (P) (a, c) and nitrogen (N) (b, d) in roots (a, b) and shoots (c, d), the shoot-to-root ratios of both elements (e, f) of tomato plants 60 days after inoculation with arbuscular mycorrhizal fungal (AMF) isolates, and subjected to different M. incognita treatments: no nematodes (NN), nematodes inoculated simultaneously with the AMF at planting (NS) or 2 weeks after AMF (NP). The AMF inoculations treatments were non-inoculated control (NM), inoculated with C. claroideum (CC), F. mosseae (FM), Gi. margarita (GM), or R. intraradices (RI). Different capital letters indicate significant differences across all treatments according to the DGC test (p < 0.05). Differences according to the DGC test (p < 0.05) for the nematode main factor effect are shown as lowercase letters at the upper left of each fungus panel when the interaction with nematode inoculation is not significant. In f, data were square root transformed to adjust for normality and homogeneity of variance. The box plot shows the 25% and 75% quartiles with median, 1.5 times interquartile range (as whiskers) and outliers. Outliers were included in all analyses. Black squares indicate the mean values of each treatment (n = 5). Statistics are presented in Table 2
In contrast to root P concentration, shoot P concentration was significantly reduced by both types of nematode application. AMF inoculation had no significant effect on this variable, neither alone nor in interaction with nematode application (Fig. 3c; Table 2). N concentration in shoots was significantly affected by nematode application and inoculation with AMF (Table 2). Inoculation with all AMF isolates reduced shoot N concentration when nematodes were applied simultaneously, but not in the other two nematode treatments (Fig. 3d). As in roots, shoot biomass was significantly negatively correlated with N concentration (r= −0.53, p = 0.0001), but not with P concentration (r= −0.005, p = 0.97) (Fig. S1c, d). The N to P ratio in shoots ranged from 21.5 to 26.5 (mean per treatment) and showed a significant interaction of AMF inoculation with nematode treatment (Table 2) that reflects the contrasting effects of AMF inoculation with and without nematode infection (Fig. S2a).
Inoculation with AMF had no overall effect on the shoot to root ratios of P and N, but significantly affected the ratios in interaction with nematode application (Fig. 3e, f; Table 2). In general, AMF inoculation tended to increase the P and N shoot to root ratios without nematodes, while an opposite tendency was observed with simultaneously inoculated nematodes.
As for the shoot dry weight, reduction of the shoot P content of non-mycorrhizal plants by nematode simultaneous infection was ameliorated by inoculation with all AMF (Fig. S2b). Shoot N content was significantly decreased by simultaneous infection with nematodes, while inoculation with AMF had no effect, neither alone nor in interaction (Fig. S2c, Table 2). Both parameters were correlated with shoot dry weight (P content: r = 0.81, p < 0.0001; N content: r = 0.75, p < 0.0001). P and N contents in roots were largely unaffected by the experimental factors, except for a significant effect of AMF on root P content, which was higher in FM than in the other inoculation treatments (Fig. S2d, e; Table 2).
Nematode galling and reproduction
In NS, a higher number of galls was observed in plants inoculated with FM and GM than in the other AMF inoculation treatments (Table 3; Fig. 4a). This increase in the number of galls was not correlated with root dry weight (r=−0.03, p = 0.98). Egg masses per gall were lower with FM, GM, and RI, as compared to NM and CC in simultaneously infected plants, whereas no differences between the AMF treatments were detected after posterior nematode inoculation (Table 3; Fig. 4b). The reproduction factor of M. incognita was not affected by AMF treatment or time of nematode application (Table 3).
Total number of galls per plant (a) and egg masses per gall (b) produced by M. incognita in tomato plants infected with the nematode simultaneously with arbuscular mycorrhizal fungi (AMF) (NS), or 2 weeks later (NP). The AMF inoculations treatments were non-inoculated control (NM), inoculated with C. claroideum (CC), F. mosseae (FM), Gi. margarita (GM), or R. intraradices (RI). Different capital letters indicate significant differences across all treatments according to the DGC test (p < 0.05). The box plot shows the 25% and 75% quartiles, the median, the whiskers (1.5 times the interquartile range), and the outliers. Outliers were included in all analyses. Black squares indicate the mean values of each treatment (n = 5). Statistics are presented in Table 3
Discussion
The main finding of this work is that all mycorrhizal isolates were able to ameliorate the drastic reduction in shoot biomass and P content caused by simultaneous infection with root-knot nematodes. This effect is consistent with some previous studies in which AMF were able to increase biomass in Meloidogyne-infected plants (Ahamad et al. 2023; Wang et al. 2023). In contrast, plants with posterior inoculation of nematodes were less affected in their growth than with simultaneous infection, even in non-mycorrhizal plants. This suggests that plants had more time to adapt to transplantation in the 2 weeks prior to nematode inoculation and therefore were less stressed by the infection. Similar results have been reported previously for a susceptible cultivar of alfalfa (Medicago sativa) (Grandison and Cooper 1986) and tomato (Talavera et al. 2001) infected with Meloidogyne spp., in which simultaneous nematode infection was more detrimental to the plants than posterior infection.
In the present study, AMF improved plant growth only when it was affected by simultaneous nematode infection. Similarly, Marro et al. (2014) reported that simultaneous AMF inoculation with the nematode Nacobbus aberrans could increase tomato growth, while no effect of AMF was observed with posterior nematode infection. In their case, however, the increase in biomass was associated with a reduction in nematode galls by AMF, contrary to our results. This variability observed in different studies reflects that the interaction between AMF and nematodes is multifactorial. For instance, the fertility of the system is one of the aspects that can cause differences between the results observed in the existing publications on the subject. It is therefore important to consider all the possible variables that can affect the interaction between nematodes and AMF in the rhizosphere and roots.
Unexpectedly, mycorrhizas did not increase the overall P concentration in tomato shoots in our experiment, even though the plants were P deficient, as indicated by their high shoot N to P ratio (Koerselman and Meuleman 1996). At the same time, the P content of the plant roots differed among experimental treatments, so it is unlikely that depletion of the entire P pool in the substrate caused this lack of mycorrhizal effects. Another possible explanation is the interplay between the direct and mycorrhizal pathways of P uptake (Smith et al. 2009; Smith and Smith 2012). The direct pathway absorbs P from the vicinity of the root epidermis and root hair cells, whereas the mycorrhizal pathway is activated by AMF root colonization and relies on specific Pi transporters that allow P to be translocated from the fungal intraradical mycelia to the root cortical cells (Smith and Smith 2011). In mycorrhizal plants, the direct pathway usually is downregulated, so that both pathways are complementary rather than additive in plant P uptake. In plants with an efficient direct pathway and low mycorrhizal responsiveness, this can lead to a lack of mycorrhizal effects on P uptake and growth even at high root colonization levels, despite a significant fungal contribution to plant P uptake, as shown specifically for tomato by Smith et al. (2004). However, as pointed out by Smith et al. (2009), the “hidden” P uptake via the mycorrhizal pathway may become “visible” when roots are damaged, and the capacity of the direct pathway is reduced by a root pathogen. This is consistent with the results of our experiment, where growth and P uptake of non-mycorrhizal plants were severely limited by nematodes, which was partially compensated by mycorrhiza.
Infection with nematodes increased P concentration in roots, which is in line with previous observations on different plants infected with Meloidogyne spp., such as tomato (Bergeson 1966; Carneiro et al. 2002; Oteifa and Elgindi 1962), pepper (Shafiee and Jenkins 1963), or Vigna radiata (Waghmare et al. 2022). Meloidogyne spp. release proteins from their pharyngeal glands to the roots and activate a cascade of immunological responses in plants, resulting in tissue lesions that form the feeding site and provide a continuous supply of nutrients to the nematode (Mitchum et al. 2013). This could explain the increase in root P and N concentrations associated with reduced growth. In addition, nematodes also reduced P translocation from roots to shoots, probably due to hypertrophy and hyperplasia of root xylem parenchyma cells due to the formation of feeding sites (Vilela et al. 2021), thus reducing P concentration in shoots. In the treatment with simultaneous nematode application, where the effect of nematodes on P partitioning was most pronounced and combined with reduced P content per plant, mycorrhiza further increased the P concentration in roots, but also P content in shoots. This suggests that AMF actually mitigated the negative effect of nematodes by supplying P to plants in a situation where direct P uptake by roots is limited. Although mycorrhizas did not increase the proportion of P translocated into the shoots, the increased supply to the roots helped to support the production of increased shoot biomass.
Similar effects as for P were found for N uptake and root-to-shoot translocation. However, some differences indicated a more important role for P than for N uptake in mycorrhizal mitigation of the damage caused by simultaneous nematode infection. The high N concentrations, as well as the highest N to P ratio in the shoots of non-mycorrhizal plants after simultaneous nematode infection, indicate that the nematode stress restricted their growth by aggravated P limitation. This interpretation is consistent with the absence of mycorrhizal effects on the total N uptake of plants simultaneously infected with nematodes. Also, the significant negative relationship of N concentration and biomass indicates growth limitation by another factor, presumably P. Plants can take up N via direct or mycorrhizal pathways, similarly to P, but contribution of the mycorrhizal pathway to the total N uptake of plants is unclear and probably less significant than in the case of P uptake (Smith and Smith 2011). Under N-deficient conditions, AMF even accumulate N in their mycelia and limit its uptake by the plant (Boussageon et al. 2022; Hodge and Fitter 2010; Ingraffia et al. 2020; Püschel et al. 2016; Treseder and Allen 2002). Therefore, it is not clear whether AMF could ameliorate N deficiency exacerbated by nematodes in a more N-limited system than in our experiment.
The described mycorrhizal effects generally were consistent among the four AMF isolates. This is remarkable in view of the significant differences in root colonization levels of the AMF isolates, because previous studies assumed that high AMF colonization should lead to elevated AMF-mediated biocontrol (e.g., Vierheilig et al. 2008). In the present work, R. intraradices and F. mosseae developed high total root colonization, while Gi. margarita and C. claroideum attained only low levels. This is in line with their root colonization characteristics as displayed in previous experiments (Blažková et al. 2021; Voříšková et al. 2017, 2019): rapid and extensive root colonization by R. irregularis, intermediate and variable root colonization ability by F. mosseae and C. claroideum isolates, and consistently low root colonization levels by Gi. margarita. Generally, F. mosseae and R. intraradices are considered to be rapid colonizers and highly infectious species, whereas Gigaspora spp. tend to produce extensive extraradical hyphae while their colonization of roots remains limited (Hart and Reader 2002; Powell et al. 2009). The similar level and mode of biotic stress alleviation despite different root colonization traits is consistent with the conclusion of Marro et al. (2022) that functional differences among AMF taxonomic groups may be smaller than previously thought.
The lack of effect of PPN infection on root colonization levels by any of the isolates contradicts previous suggestions that these nematodes may stimulate AMF colonization by altering the composition of root exudates to increase signals that act as a ‘cry for help’ (Rolfe et al. 2019). Conversely, competition for space and photosynthates has been hypothesized to reduce mycorrhizal colonization in the presence of Meloidogyne (da Silva Campos 2020; De Sá 2020). No effect of nematodes on AMF root colonization also has been reported (Anjos et al. 2010; Azcón-Aguilar and Barea 1996), suggesting that there is no general rule for this interaction, which may depend on the identity of AMF, PPN, and experimental conditions. Nevertheless, we found interesting effects of nematode infection on the formation of specific fungal structures: it significantly increased the frequency of vesicles in root colonization by C. claroideum and reduced the number of arbuscules by F. mosseae and Gi. margarita. Arbuscules are intracellular branched hyphal structures for nutrient exchange between the two symbionts (Tian et al. 2013), whereas vesicles are lipid reserves for fungal maintenance (Montero et al. 2019), formed only by some species. It has been suggested that the reduction of arbuscules and allocation of energy to fungal storage (vesicles) and reproductive structures (spores) is indicative for less mutualistic mycorrhizas (Buil et al. 2023; Cabello 1997; Johnson et al. 1997). Consequently, nematodes might have weakened the cooperation between the plant and its mycorrhizal symbionts, with AMF species responding differently to this stress. Interestingly, R. irregularis was the AMF species least responsive to nematode infection by altering its root colonization structures. Previously, this species was reported to be a generalist in terms of reliably supplying nutrients to its host plant under a range of nutrient conditions (Boussageon et al. 2022). Our results suggest that its interaction with the host plant also may be more robust to plant stress than that of the other AMF species tested in the experiment.
Surprisingly, the improved growth of mycorrhizal plants was not associated with a reduction in nematode development or reproduction. Similar results have been reported previously (Rodriguez-Heredia et al. 2020; Wang et al. 2023). Contrary to our expectations, more nematodes were able to form galls in the roots of F. mosseae and Gi. margarita than in control plants, indicating a higher level of nematode infection. The reason for this effect is unclear based on the available data. It is possible that this is related to the life cycles of these AMF isolates, such as a slower initial development of root colonization. However, this hypothesis needs to be examined.
Interestingly, we observed that not all galls contained egg masses at plant harvest, either because the eggs already hatched or they were not produced yet. However, the number of egg masses per gall was lower in F. mosseae, Gi. margarita, and R. intraradices than in non-mycorrhizal plants, suggesting that the nematodes were able to penetrate the roots, but a smaller proportion of them were able to reach the reproductive stage. In the case of the posterior nematode inoculation, even though the differences were non-significant, AMF plants tended to have fewer galls per plant, but more egg masses per gall than the non-mycorrhizal plants, which created an opposite pattern compared to simultaneous infection (see Fig. 4). This may suggest different mechanisms of the AMF protective effects depending on the timing of the nematode infection: simultaneous root colonization by both organisms may not reduce gall development although it affects the nematode ability to reproduce, while AMF root colonization prior to nematode infection may reduce nematode gall establishment.
Conclusion
Our study demonstrated alleviation of nematode-induced stress by four AMF with different root colonization traits, which consistently improved plant P uptake and growth. Regarding the possible mechanisms of this effect, direct competition for space and carbon from roots (Schouteden et al. 2015) was not a likely cause in our study, as root penetration by nematodes was not significantly reduced in the presence of AMF, neither after simultaneous nor after posterior inoculation. Also, the number of galls was not related to the degree of root colonization by the different isolates.
Another mode of action of AMF against nematodes might be to increase plant tolerance to nematode attack. This was not exactly observed in our experiment as AMF did not improve the growth of non-infected plants per se. However, the alleviation of nematode-induced stress by AMF was clearly associated with improved P uptake. The fact that this stress alleviation was mediated by all AMF species, irrespective of the level of root colonization, highlights the importance of this mechanism. Considering the lack of plant benefits from AMF in the absence of nematodes, our results emphasize the need to focus on the interaction between stress and nutritional benefits of mycorrhizal fungi, which may be an important factor in stress alleviation. Indeed, it has been seen that P levels clearly affect plant defense responses to pathogens and herbivores (Chan et al. 2021). Mediation of stress resistance by the nutritional benefits of mycorrhizas also has been clearly demonstrated for other types of stress such as drought (Püschel et al. 2021), salinity (Qin et al. 2021), or low temperature (Chen et al. 2013). While non-nutritional benefits, such as induction of plant defenses by AMF, could be another explanation for our results, they were not explicitly addressed in our experiment. Further studies that focus on the variability among AMF species and specific pathways for the induction of resistance or tolerance will be important to ascertain how different AMF species affect the plant balance between growth and defense and to further elucidate the biological control potential of different AMF in soils.
References
Abd-Elgawad MMM (2021) Optimizing safe approaches to manage plant-parasitic nematodes. Plants 10:1911. https://doi.org/10.3390/plants10091911
Ahamad L, Bhat AH, Kumar H, Rana A, Hasan MN, Ahmed I, Ahmed S, Machado RAR, Ameen F (2023) From soil to plant: strengthening carrot defenses against Meloidogyne incognita with vermicompost and arbuscular mycorrhizal fungi biofertilizers. Front Microbiol 14:1206217. https://doi.org/10.3389/fmicb.2023.1206217
Ammiraju JS, Veremis JC, Huang X, Roberts PA, Kaloshian I (2003) The heat-stable root-knot nematode resistance gene Mi-9 from Lycopersicon peruvianum is localized on the short arm of chromosome 6. Theor Appl Genet 106:478e484
Anjos ÉCTD, Cavalcante UMT, Gonçalves DMC, Pedrosa EMR, Santos VFD, Maia LC (2010) Interactions between an arbuscular mycorrhizal fungus (Scutellospora heterogama) and the root-knot nematode (Meloidogyne incognita) on sweet passion fruit (Passiflora alata). Braz Arch Biol Technol 53:801–809
Azcon-Aguilar C, Barea JM (1996) Arbuscular mycorrhizas and biological control of soilborne plant pathogens, an overview of the mechanisms involved. Mycorrhiza 6:457–464
Banuelos J, Alarcón A, Larsen J, Cruz-Sánchez S, Trejo D (2014) Interactions between arbuscular mycorrhizal fungi and Meloidogyne incognita in the ornamental plant Impatiens balsamina. J Soil Sci Plant Nutr 14:63–74
Bergeson GB (1966) Mobilization of minerals to the infection site of root knot nematodes. Phytopathology 56:1287–1289
Berruti A, Lumini E, Balestrini R, Bianciotto V (2016) Arbuscular mycorrhizal fungi as natural biofertilizers: let’s benefit from past successes. Front Microbiol 6:1559
Blažková A, Jansa J, Püschel D, Vosatka M, Janoušková M (2021) Is mycorrhiza functioning influenced by the quantitative composition of the mycorrhizal fungal community? Soil Biol Biochem 157:108249. https://doi.org/10.1016/j.soilbio.2021.108249
Boussageon R, Marro N, Janoušková M, Brulé D, Wipf D, Courty PE (2022) The fine-tuning of mycorrhizal pathway in Sorghum depends on both nitrogen-phosphorus availability and the identity of the fungal partner. Plant Cell Environ 45:3354–3366. https://doi.org/10.1111/pce.14426
Buil PA, Jansa J, Blažková A, Holubík O, Duffková R, Rozmoš M, Püschel D, Kotianová M, Janoušková M (2023) Infectivity and symbiotic efficiency of native arbuscular mycorrhizal fungi from high-input arable soils. Plant Soil 482:627–645. https://doi.org/10.1007/s11104-022-05715-8
Cabello MN (1997) Hydrocarbon pollution: its effect on native arbuscular mycorrhizal fungi (AMF). FEMS Microbiol Ecol 22:233–236
Carneiro RG, Mazzafera P, Ferraz ICCB, Muraoka T, Trevelin PCO (2002) Uptake and translocation of nitrogen, phosphorus and calcium in soybean infected with Meloidogyne incognita and M. javanica. Fitopatol Bras 27:141–150
Carrara JE, Heller WP (2022) Arbuscular mycorrhizal species vary in their impact on nutrient uptake in sweet corn (Zea mays) and butternut squash (Cucurbita moschata). Front Agron 4:1040054
Castagnone-Sereno P, Danchin EGJ, Perfus-Barbeoch L, Abad P (2013) Diversity and evolution of Root-Knot nematodes, Genus Meloidogyne: New insights from the genomic era. Annu Rev Phytopathol 51:203–220. https://doi.org/10.1146/annurev-phyto-082712-102300
Castillo P, Nico AI, Azcón-Aguilar C, Del Río Rincón C, Calvet C, Jiménez-Díaz RM (2006) Protection of olive planting stocks against parasitism of root-knot nematodes by arbuscular mycorrhizal fungi. Plant Pathol 55:705–713. https://doi.org/10.1111/j.1365-3059.2006.01400.x
Chan C, Liao YY, Chiou TJ (2021) The impact of phosphorus on plant immunity. Plant Cell Physiol 62(4):582–589
Chen S, Jin W, Liu A, Zhang S, Liu D, Wang F, Lin X, He C (2013) Arbuscular mycorrhizal fungi (AMF) increase growth and secondary metabolism in cucumber subjected to low temperature stress. Sci Hortic 160:222–229
da Silva Campos MA (2020) Bioprotection by arbuscular mycorrhizal fungi in plants infected with Meloidogyne nematodes: a sustainable alternative. Crop Prot 135:105203
de Almeida GQ, de Oliveira Silva J, Copati MGF, de Oliveira Dias F, dos Santos MC (2020) Tomato breeding for disease resistance. Multi-Sci J 3(3):8–16
De Sá CSB, Campos MAS (2020) Arbuscular mycorrhizal fungi decrease Meloidogyne Enterolobii infection of Guava seedlings. J Helminthol 94:e183
Decraemer W, Hunt DJ (2006) Structure and classification. Plant nematology. CABI, Wallingford UK, pp 3–32
Di Rienzo JA, Guzmán AW, Casanoves F (2002) A multiple-comparisons method based on the distribution of the root node distance of a binary tree. J Agric Biol Environ Stat 7:129–142. https://doi.org/10.1198/10857110260141193
Diagne N, Ngom M, Djighaly PI, Fall D, Hocher V, Svistoonoff S (2020) Roles of arbuscular mycorrhizal fungi on plant growth and performance: importance in biotic and abiotic stressed regulation. Diversity 12:370
Dowarah B, Gill SS, Agarwala N (2022) Arbuscular mycorrhizal fungi in conferring tolerance to biotic stresses in plants. J Plant Growth Regul 41:1429–1444. https://doi.org/10.1007/s00344-021-10392-50
Escobar C, Barcala M, Cabrera J, Fenoll C (2015) Overview of root-knot nematodes and giant cells. Advances in botanical research, vol 73. Academic, pp 1–32
Gao X, Starr J, Göbel C, Engelberth J, Feussner I, Tumlinson J, Kolomiets M (2008) Maize 9-lipoxygenase ZmLOX3 controls development, root-specific expression of defense genes, and resistance to root-knot nematodes. Mol Plant Microbe Interact 21:98–109
Grandison GS, Cooper KM (1986) Interaction of vesicular-arbuscular mycorrhizae and cultivars of alfalfa susceptible and resistant to Meloidogyne hapla. J Nematol 18:141
Gutiérrez-Gutiérrez C, Palomares-Rius JE, Jiménez-Díaz RM, Castillo P (2011) Host suitability of Vitis rootstocks to root-knot nematodes (Meloidogyne spp.) and the dagger nematode Xiphinema index, and plant damage caused by infections. Plant Pathol 60:575–585. https://doi.org/10.1111/j.1365-3059.2010.02404.x
Hart MM, Reader RJ (2002) Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol 153:335–344. https://doi.org/10.1046/j.0028-646X.2001.00312.x
Hartig F, Lohse L (2020) Residual diagnostics for hierarchical (multi-level/mixed) regression models. Package ‘DHARMa’ Version 0.3.3.0. https://CRAN.R-project.org/package=DHARMa
Herrera-Parra E, Ramos-Zapata J, Basto-Pool C, Cristóbal-Alejo J (2021) Sweet pepper (Capsicum annuum) response to the inoculation of native arbuscular mycorrhizal fungi and the parasitism of root-knot Meloidogyne incognita. Revista Bio Ciencias 8:e982. https://doi.org/10.15741/revbio.08.e982
Hewitt EJ (1966) Sand and water culture methods used in the study of plant nutrition. Technical communication no. 22. Commonwealth Agriculture Bureau
Hodge A, Fitter AH (2010) Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc Natl Acad Sci USA 107:13754–13759
Hussey RS, Barker KR (1973) A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis Rep 57:1025–1028
InfoStat versión 2011 (2011) Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. http://www.infostat.com.ar
Ingraffia R, Amato G, Sosa-Hernández MA, Frenda AS, Rillig MC, Giambalvo D (2020) Nitrogen type and availability drive mycorrhizal effects on wheat performance, nitrogen uptake and recovery, and production sustainability. Front Plant Sci 11:760
Jansa J, Smith FA, Smith SE (2008) Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol 177:779–789
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol 135:575–585. https://doi.org/10.1046/j.1469-8137.1997.00729.x
Jones JT, Haegeman A, Danchin EG, Gaur HS, Helder J, Jones MG, Kikuchi T, Manzanilla-López R, Palomares-Rius JE, Wesemael WM, Perry RN (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14:946–961
Klironomos JN, Hart MM (2002) Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza 12:181–184. https://doi.org/10.1007/s00572-002-0169-6
Koerselman W, Meuleman AFM (1996) The vegetation N: P ratio: A new tool to detect the nature of nutrient limitation. J Appl Ecol 33:1441–1450
Koske RE, Gemma JN (1989) A modified procedure for staining roots to detect VA mycorrhizas. Mycol Res 92:486–505
López-Ráez JA, Verhage A, Fernandez I, García JM, Azcon-Aguilar C, Flors V, Pozo M (2010) Hormonal and transcriptional profiles highlight common and differential host responses to arbuscular mycorrhizal fungi and the regulation of the oxylipin pathway. J Exp Bot 61:2589–2601
Marro N, Caccia M, Doucet ME, Cabello M, Becerra A, Lax P (2018) Mycorrhizas reduce tomato root penetration by false root–knot nematode Nacobbus aberrans. Appl Soil Ecol 124:262–265
Marro N, Grilli G, Soteras F, Caccia M, Longo S, Cofré N, Borda V, Burni M, Janoušková M, Urcelay C (2022) The effects of arbuscular mycorrhizal fungal species and taxonomic groups on stressed and unstressed plants: A global meta-analysis. New Phytol 235:320–332
Marro N, Lax P, Doucet ME, Cabello M, Becerra A (2014) Use of the arbuscular mycorrhizal fungus Glomus intraradices as biological control agent of the nematode Nacobbus aberrans parasitizing tomato. Braz Arch Biol Technol 57:668–674
Marulanda A, Azcon R, Ruiz-Lozano JM (2003) Contribution of six arbuscular mycorrhizal fungal isolates to water uptake by Lactuca sativa plants under drought stress. Physiol Plant 119:526–533. https://doi.org/10.1046/j.1399-3054.2003.00196.x
Mc Gonigle TP, Miller MH, Evans DG et al (1990) A new method which gives and objective measure of colonization of roots by vesicular arbuscular mycorrhizal fungi. New Phytol 115:495–501. https://doi.org/10.1111/j.1469-8137.1990.tb00476.x
Mensah JA, Koch AM, Antunes PM et al (2015) High functional diversity within species of arbuscular mycorrhizal fungi is associated with differences in phosphate and nitrogen uptake and fungal phosphate metabolism. Mycorrhiza 25:533–546. https://doi.org/10.1007/S00572-015-0631-X
Mitchum MG, Hussey RS, Baum TJ, Wang X, Elling AA, Wubben M, Davis EL (2013) Nematode effector proteins: an emerging paradigm of parasitism. New Phytol 199:879–894
Molinari S, Leonetti P (2019) Bio-control agents activate plant immune response and prime susceptible tomato against root-knot nematodes. PLoS ONE 14(12):e0213230. https://doi.org/10.1371/journal.pone.021323
Montero H, Choi J, Paszkowski U (2019) Arbuscular mycorrhizal phenotyping: the dos and don’ts. New Phytol 221:1182–1186. https://doi.org/10.1111/nph.15489
Munkvold L, Kjøller R, Vestberg M, Rosendahl S, Jakobsen I (2004) High functional diversity within species of arbuscular mycorrhizal fungi. New Phytol 164:357–364. https://doi.org/10.1111/j.1469-8137.2004.01169.x
Murphy J, Riley JP (1962) A modified single solution method the determination of phosphate. Anal Chim Acta 27:31–36
Oteifa BA, Elgindi DM (1962) Influence of parasitic duration of Meloidogyne javanica (Treub) on host nutrient uptake. Nematologica 8:216–220
Perry R, Moens M (2011) Introduction to plant-parasitic nematodes; modes of parasitism. Genomics and molecular genetics of plant-nematode interactions. Springer Netherlands, pp 3–20
Phani V, Khan MR, Dutta TK (2021) Plant-parasitic nematodes as a potential threat to protected agriculture: current status and management options. Crop Prot 144:105573
Powell JR, Parrent JL, Hart MM, Klironomos JN, Rillig MC, Maherali H (2009) Phylogenetic trait conservatism and the evolution of functional trade-offs in arbuscular mycorrhizal fungi. Proc Royal Soc B Biol Sci 276:4237–4245
Pozo MJ, Azcon-Aguilar C (2007) Unraveling mycorrhiza-induced resistance. Curr Opin Plant Biol 10:393–398. https://doi.org/10.1016/j.pbi.2007.05.004. PMID: 17658291
Püschel D, Bitterlich M, Rydlová J, Jansa J (2021) Drought accentuates the role of mycorrhiza in phosphorus uptake. Soil Biol Biochem 157:1–11. https://doi.org/10.1016/j.soilbio.2021.108243
Püschel D, Janoušková M, Hujslová M, Slavíková R, Gryndlerová H, Jansa J (2016) Plant–fungus competition for nitrogen erases mycorrhizal growth benefits of Andropogon gerardii under limited nitrogen supply. Ecol Evol 6:4332–4346
Qin WJ, Yan HY, Zou BY, Guo RZ, Ci DW, Tang ZH, Zou XX, Zhang XJ, Yu XN, Wang YF, Si T (2021) Arbuscular mycorrhizal fungi alleviate salinity stress in peanut: evidence from pot-grown and field experiments. Food Energy Secur 10:e314. https://doi.org/10.1002/fes3.314
R Development Core Team (2011) R: A language and environment for statistical computing
Rodriguez-Heredia M, Djian-Caporalino C, Ponchet M, Lapeyre L, Canaguier R, Fazari A, Marteu N, Industri B, Offroy-Chave M (2020) Protective effects of mycorrhizal association in tomato and pepper against Meloidogyne incognita infection, and mycorrhizal networks for early mycorrhization of low mycotrophic plants. Phytopathol Mediterr 59:377–384. https://doi.org/10.14601/Phyto-11637
Rolfe SA, Griffiths J, Ton J (2019) Crying out for help with root exudates: adaptive mechanisms by which stressed plants assemble health-promoting soil microbiomes. Curr Opin Microbiol 49:73–82. https://doi.org/10.1016/j.mib.2019.10.003Epub Nov 13. PMID: 31731229
Säle V, Palenzuela J, Azcón-Aguilar C, Sánchez-Castro I, da Silva GA, Seitz B, Seitz B, Sieverding E, Heijden MG, vander, Oehl F (2021) Ancient lineages of arbuscular mycorrhizal fungi provide little plant benefit. Mycorrhiza 31:559–576. https://doi.org/10.1007/s00572-021-01042-5
Schouteden N, De Waele D, Panis B, Vos CM (2015) Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: a review of the mechanisms involved. Front Microbiol 6:1280. https://doi.org/10.3389/fmicb.2015.01280
Shafiee MF, Jenkins WR (1963) Host-parasite relationships of Capsicum frutescens and Pratylenchus penetrans, Meloidogyne incognita acrita, and M. hapla. Phytopathol 53:325–328
Sikes BA, Cottenie K, Klironomos JN (2009) Plant and fungal identity determines pathogen protection of plant roots by arbuscular mycorrhizas. J Ecol 97:1274–1280. https://doi.org/10.1111/j.13652745.2009.01557.x
Smith FA, Grace EJ, Smith SE (2009) More than a carbon economy: Nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytol 182:347–358. https://doi.org/10.1111/j.1469-8137.2008.02753.x
Smith SE, Smith FA (2011) Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol 62:227–250. https://doi.org/10.1146/annurev-arplant-042110-103846. PMID: 21391813
Smith SE, Smith FA (2012) Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia 104:1–13. https://doi.org/10.3852/11-229
Smith SE, Smith FA, Jakobsen I (2004) Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol 162:511–524. https://doi.org/10.1111/j.1469-8137.2004.01039.x
Subedi S, Thapa B, Shrestha J (2020) Root-knot nematode (Meloidogyne incognita) and its management: A review. J Agric Nat Resour 3(2):21–31
Talavera M, Itou K, Mizukubo T (2001) Reduction of nematode damage by root colonization with arbuscular mycorrhiza (Glomus spp.) in tomato-Meloidogyne incognita (Tylenchida: Meloidogynidae) and carrot-Pratylenchus penetrans (Tylenchida: Pratylenchidae) pathosystems. Appl Entomol Zool 36:387–392
Terry V, Kokkoris V, Villeneuve-Laroche M, Turcu B, Chapman K, Cornell C, Zheng Z, Stefani F, Corradi N (2023) Mycorrhizal response of Solanum tuberosum to homokaryotic versus dikaryotic arbuscular mycorrhizal fungi. Mycorrhiza 33:333–344. https://doi.org/10.1007/s00572-023-01123-7
Tian M, Chen YL, Li M, Liu RJ (2013) Structure and function of arbuscular mycorrhiza: A review. J Appl Ecol 24:2369–2376
Treseder KK, Allen MF (2002) Direct nitrogen and phosphorus limitation of arbuscular mycorrhizal fungi: a model and field test. New Phytol 155:507–515
Turrini A, Avio L, Giovannetti M, Agnolucci M (2018) Functional complementarity of arbuscular mycorrhizal fungi and associated microbiota: The challenge of translational research. Front Plant Sci 9:1407. https://doi.org/10.3389/fpls.2018.01407
Vierheilig H, Steinkellner S, Khaosaad T (2008) The biocontrol effect of mycorrhization on soilborne fungal pathogens and the autoregulation of the AM symbiosis: one mechanism, two effects? Mycorrhiza. Springer-, Berlin, pp 307–320. doi: https://doi.org/10.1007/978-3-540-78826-3_15
Vilela RMIF, Kuster VC, Magalhães TA, Moraes CA, Paula Filho AC, Oliveira DC, Moench (2021) Impact of Meloidogyne incognita (nematode) infection on root tissues and cell wall composition of okra (Abelmoschus esculentus L. Moench, Malvaceae). Protoplasma 258:979–990. https://doi.org/10.1007/s00709-021-01618-0
Voříšková A, Jansa J, Püschel D, Krüger M, Cajthaml T, Vosátka M, Janoušková M (2017) Real-time PCR quantification of arbuscular mycorrhizal fungi: does the use of nuclear or mitochondrial markers make a difference? Mycorrhiza 27:577–585. https://doi.org/10.1007/s00572-017-0777-9
Voříšková A, Jansa J, Püschel D, Vosátka M, Šmilauer P, Janoušková M (2019) Abiotic contexts consistently influence mycorrhiza functioning independently of the composition of synthetic arbuscular mycorrhizal fungal communities. Mycorrhiza 29:127–139. https://doi.org/10.1007/s00572-018-00878-8
Vos C, Claerhout S, Mkandawire R, Panis B, De Waele D, Elsen A (2012a) Arbuscular mycorrhizal fungi reduce root-knot nematode penetration through altered root exudation of their host. Plant Soil 354:335–345
Vos C, Tesfahun AN, Panis B, De Waele D, Elsen A (2012b) Arbuscular mycorrhizal fungi induce systemic resistance in tomato against the sedentary nematode Meloidogyne incognita and the migratory nematode Pratylenchus penetrans. Appl Soil Ecol 61:1–6
Vos C, Schouteden N, Van Tuinen D, Chatagnier O, Elsen A, De Waele D, Panis B, Gianinazzi-Pearson V (2013) Mycorrhiza-induced resistance against the root–knot nematode Meloidogyne incognita involves priming of defense gene responses in tomato. Soil Biol Bioch 60:45–54
Waghmare C, Singh P, Paul S, Sharma HK (2022) Influence of root-knot nematode, Meloidogyne incognita (Kofoid & White) Chitwood infection on different plant growth parameters in Mungbean, Vigna radiata (L.) Wilczek. Indian J Exp Biol 60:351–359
Wang L, Chen X, Tang Z (2023) Arbuscular mycorrhizal symbioses improved biomass allocation and reproductive investment of cherry tomato after root-knot nematodes infection. Plant Soil 482:513–527. https://doi.org/10.1007/s11104-022-05708
Zbíral J (1994) Analýza rostlinného materiálu. Jednotné pracovní postupy [Analysis of plant material. Unified techniques]. Státní kontrolní a zkušební ústav zemědělský, Brno
Acknowledgements
The work was financially supported by project no. CZ.02.2.69/0.0/0.0/18_054/0014676 of Ministry of Education, Youth and Sports of the Czech Republic, co-financed by the European Union; Czech Science Foundation, project no. 23-05453 S; and the long-term research development program of Czech Academy of Sciences RVO 67985939.Technical assistance of Soňa Zvolenská is greatly acknowledged.
Funding
Open access publishing supported by the National Technical Library in Prague. The research leading to these results received funding from project no. CZ.02.2.69/0.0/0.0/18_054/0014676 of Ministry of Education, Youth and Sports of the Czech Republic co-financed by the European Union; Czech Science Foundation, project no. 23-05453 S; and the long-term research development program of Czech Academy of Sciences RVO 67985939.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by M. C. and V.N. The first draft of the manuscript was written by M.C., N. M., and M. J. All authors commented on the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
Martina Janoušková is a member of the Editorial Board. The authors have no other relevant financial or non-financial interests to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Caccia, M., Marro, N., Novák, V. et al. Divergent colonization traits, convergent benefits: different species of arbuscular mycorrhizal fungi alleviate Meloidogyne incognita damage in tomato. Mycorrhiza 34, 145–158 (2024). https://doi.org/10.1007/s00572-024-01139-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-024-01139-7