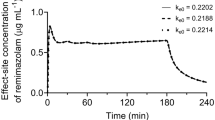
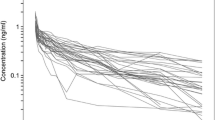
Abstract
Background
Remimazolam besylate is a novel short-acting benzodiazepine. An appropriate pharmacokinetic model of remimazolam is desirable in anesthesia practice. The aim of the study was to develop a pharmacokinetic model using plasma samples from patients anesthetized with remimazolam. Influence of patient characteristics, context-sensitive decrement-times, and dose regimens were also examined.
Methods
Data were obtained from four trials on patients, and seven trials on healthy volunteers. The characteristics of 416 male and 246 female subjects were as follows: age, 18–93 years; body weight, 34–149 kg; and American Society of Anesthesiologists physical status (ASA-PS), I-IV. 2231 arterial and 3200 venous samples were used for the final model. The equilibration rate constant between arterial plasma and effect-site was estimated using the concept of time to peak effect. The final model was used to generate context-sensitive decrement times and dose regimens for general anesthesia.
Results
A three-compartment model plus virtual venous compartment with allometric scaling of adjusted body weight (ABW), age, sex, and ASA-PS as covariates were selected as the final model. Elimination clearance was lower in males, and in subjects with higher ABW and ASA-PS scores. Approximately 10% or 20% higher dose rate was necessary in females than in males or ASA-PS I/II than III/IV patient. The context-sensitive half-time for effect-site concentration in a 55-year-old, 70-kg, 170-cm male or female ASA-PS I/II patient after > 6-h infusion was 16.7 or 15.9 min.
Conclusion
Remimazolam pharmacokinetic model for general anesthesia was successfully developed. ABW, ASA-PS, and sex has a considerable impact on the remimazolam concentration.






Similar content being viewed by others
References
Birgenheier NM, Stuart AR, Egan TD. Soft drugs in anesthesia: remifentanil as prototype to modern anesthetic drug development. Curr Opin Anaesthesiol. 2020;33:499–505.
Schüttler J, Eisenried A, Lerch M, Fechner J, Jeleazcov C, Ihmsen H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part I pharmacokinetics and clinical pharmacodynamics. Anesthesiol. 2020;132:636–51.
Eisenried A, Schüttler J, Lerch M, Ihmsen H, Jeleazcov C. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part II Pharmacodynamics of Electroencephalogram Effects. Anesthesiol. 2020;132:652–66.
Rex DK, Bhandari R, Desta T, DeMicco MP, Schaeffer C, Etzkorn K, Barish CF, Pruitt R, Cash BD, Quirk D, Tiongco F, Sullivan S, Bernstein D. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88:427-37.e6.
Borkett KM, Riff DS, Schwartz HI, Winkle PJ, Pambianco DJ, Lees JP, Wilhelm-Ogunbiyi K. A phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg. 2015;120:771–80.
Worthington MT, Antonik LJ, Goldwater DR, Lees JP, Wilhelm-Ogunbiyi K, Borkett KM, Mitchell MC. A phase Ib, dose-finding study of multiple doses of remimazolam (CNS 7056) in volunteers undergoing colonoscopy. Anesth Analg. 2013;117:1093–100.
Wiltshire HR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part II. Population pharmacokinetic and pharmacodynamic modeling and simulation. Anesth Analg. 2012;115:284–96.
Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part I. Safety, efficacy, and basic pharmacokinetics. Anesth Analg. 2012;115:274–83.
Doi M. Remimazolam. J Japan Soc Clin Anesth. 2014. https://doi.org/10.2199/jjsca.34.860.
Doi M, Hirata N, Suzuki T, Morisaki H, Morimatsu H, Sakamoto A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. 2020;34(4):491–501.
Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34:543–53.
Masui K. Remimazolam besilate, a benzodiazepine, has been approved for general anesthesia!! J Anesth. 2020;34:479–82.
Sneyd JR. Remimazolam: new beginnings or just a me-too? Anesth Analg. 2012;115:217–9.
Bennett SN, McNeil MM, Bland LA, Arduino MJ, Villarino ME, Perrotta DM, Burwen DR, Welbel SF, Pegues DA, Stroud L, Zeitz PS, Jarvis WR. Postoperative infections traced to contamination of an intravenous anesthetic propofol. N Engl J Med. 1995;333:147–54.
Struys MMRF, Absalom AR, Shafer SL. Intravenous Drug Delivery System. In: Anesthesia M, editor. Miller RD. Philadelphia: Elsevier Saunders; 2015. p. 919–57.
Eleveld DJ, Proost JH, Cortinez LI, Absalom AR, Struys MM. A general purpose pharmacokinetic model for propofol. Anesth Analg. 2014;118:1221–37.
Eleveld DJ, Colin P, Absalom AR, Struys MMRF. Pharmacokinetic–pharmacodynamic model for propofol for broad application in anaesthesia and sedation. Br J Anaesth. 2018;120:942–59.
Zhou J, Leonowens C, Ivaturi VD, Lohmer LL, Curd L, Ossig J, Schippers F, Petersen K-U, Stoehr T, Schmith V. Population pharmacokinetic/pharmacodynamic modeling for remimazolam in the induction and maintenance of general anesthesia in healthy subjects and in surgical subjects. J Clin Anesth. 2020;66: 109899.
Chiou WL. The phenomenon and rationale of marked dependence of drug concentration on blood sampling site. Clin Pharmacokinet. 1989;17:175–99.
Tuk B, Herben VM, Mandema JW, Danhof M. Relevance of arteriovenous concentration differences in pharmacokinetic-pharmacodynamic modeling of midazolam. J Pharmacol Exp Ther. 1998;284:202–7.
Peacock JE, Blackburn A, Sherry KM, Reilly CS. Arterial and jugular venous bulb blood propofol concentrations during induction of anesthesia. Anesth Analg. 1995;80:1002–6.
Stöhr T, Colin PJ, Ossig J, Pesic M, Borkett K, Winkle P, Struys MMRF, Schippers F. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br J Anaesth. 2021;127:415–23.
Kleiman RB, Darpo B, Thorn M, Stoehr T, Schippers F. Potential strategy for assessing QT/QTc interval for drugs that produce rapid changes in heart rate: electrocardiographic assessment of the effects of intravenous remimazolam on cardiac repolarization. Br J Clin Pharmacol. 2020;86:1600–9.
Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44:1051–65.
Green B, Duffull SB. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol. 2004;58:119–33.
West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997;276:122–6.
Beal SL. Ways to Fit a PK Model with Some Data Below the Quantification Limit. J Pharmacokinet Pharmacodyn. 2001;28:481–504.
Glass PS, Shafer S, Reves JG. Intravenous drug delivery systems. In: Miller RD, editor. Miller’s anesthesia. 6th ed. Pennsylvania, USA: Elsevier; 2004. p. 439–80.
Varvel JR, Donoho DL, Shafer SL. Measuring the predictive performance of computer-controlled infusion pumps. J Pharmacokinet Biopharm. 1992;20:63–94.
Minto CF, Schnider TW, Gregg KM, Henthorn TK, Shafer SL. Using the time of maximum effect site concentration to combine pharmacokinetics and pharmacodynamics. Anesthesiology. 2003;99:324–33.
Hughes MA, Glass PS, Jacobs JR. Context-sensitive half-time in multicompartment pharmacokinetic models for intravenous anesthetic drugs. Anesthesiology. 1992;76:334–41.
Eger EI 2nd, Shafer SL. Tutorial: context-sensitive decrement times for inhaled anesthetics. Anesth Analg. 2005;101:688–96.
Sheng XY, Liang Y, Yang XY, Li LE, Ye X, Zhao X, Cui YM. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur J Clin Pharmacol. 2020;76:383–91.
Vree TB, Dammers E, Ulc I, Horkovics-Kovats S, Ryska M, Merkx I. Male-female differences in the plasma, liver and tissue esterase hydrolysis of lovastatin in healthy volunteers after a single oral dose. Clin Drug Investig. 2002;22:181–90.
Sinha J, Duffull SB, Green B, Al-Sallami HS. Evaluating the relationship between lean liver volume and fat-free mass. Clin Pharmacokinet. 2020;59:475–83.
Cortinez LI, De la Fuente N, Eleveld DJ, Oliveros A, Crovari F, Sepulveda P, Ibacache M, Solari S. Performance of propofol target-controlled infusion models in the obese: pharmacokinetic and pharmacodynamic analysis. Anesth Analg. 2014;119:302–10.
Zhou J, Curd L, Lohmer LL, Ossig J, Schippers F, Stoehr T, Schmith V. Population pharmacokinetics of remimazolam in procedural sedation with nonhomogeneously mixed arterial and venous concentrations. Clin Transl Sci. 2021;14:326–34.
Nelson RY, Bretz B, Egan TD. Prolonged apnea after remifentanil. J Clin Anesth. 2007;19:60–3.
Acknowledgements
We thank Akito Ishikawa and Rieko Ichihara (Data Science Department, Mundipharma K.K., Tokyo, Japan), who cross-checked the spread sheet containing the data for pharmacokinetic analysis.
We received a professional English editing service from Cactus Communications. K.K., Tokyo, Japan.
Funding
This study was mainly funded by a research grant from Mundipharma K.K., Tokyo, Japan, and was partially funded by the Department of Anesthesiology, Showa University School of Medicine, Japan.
Author information
Authors and Affiliations
Contributions
Kenichi Masui: This author contributed to the study design, data analysis and interpretation, and manuscript preparation. Marija Pesic, Thomas Stöhr and Tomohiro Tonai: These authors contributed to data preparation and interpretation as well as manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
Kenichi Masui was a consultant/advisor for Mundipharma K.K., Tokyo, Japan, and has been awarded a research grant for this study and has received payment for delivering domestic lectures from Mundipharma. Additionally, he is an Editor of Journal of Anesthesia, and an Editorial Board of JA Clinical Reports. Thomas Stöhr and Marija Pesic are employees of PAION Deutschland GmbH, Aachen, Germany. PAION is the developer of remimazolam besylate. Tomohiro Tonai is an employee of Mundipharma K.K., Tokyo, Japan. Mundipharma have an exclusive license and rights for development and commercialization of remimazolam in Japan.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Masui, K., Stöhr, T., Pesic, M. et al. A population pharmacokinetic model of remimazolam for general anesthesia and consideration of remimazolam dose in clinical practice. J Anesth 36, 493–505 (2022). https://doi.org/10.1007/s00540-022-03079-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-022-03079-y