Abstract
Purpose
Rocuronium concentration prediction using pharmacokinetic (PK) models would be useful for controlling rocuronium effects because neuromuscular monitoring throughout anesthesia can be difficult. This study assessed whether six different compartmental PK models developed from data obtained after bolus administration only could predict the measured plasma concentration (Cp) values of rocuronium delivered by bolus followed by continuous infusion.
Methods
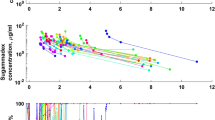
Rocuronium Cp values from 19 healthy subjects who received a bolus dose followed by continuous infusion in a phase III multicenter trial in Japan were used retrospectively as evaluation datasets. Six different compartmental PK models of rocuronium were used to simulate rocuronium Cp time course values, which were compared with measured Cp values. Prediction error (PE) derivatives of median absolute PE (MDAPE), median PE (MDPE), wobble, divergence absolute PE, and divergence PE were used to assess inaccuracy, bias, intra-individual variability, and time-related trends in APE and PE values.
Results
MDAPE and MDPE values were acceptable only for the Magorian and Kleijn models. The divergence PE value for the Kleijn model was lower than −10 %/h, indicating unstable prediction over time. The Szenohradszky model had the lowest divergence PE (−2.7 %/h) and wobble (5.4 %) values with negative bias (MDPE = −25.9 %). These three models were developed using the mixed-effects modeling approach. The Magorian model showed the best PE derivatives among the models assessed.
Conclusions
A PK model developed from data obtained after single-bolus dosing can predict Cp values during bolus and continuous infusion. Thus, a mixed-effects modeling approach may be preferable in extrapolating such data.



Similar content being viewed by others
References
Maybauer DM, Geldner G, Blobner M, Puhringer F, Hofmockel R, Rex C, Wulf HF, Eberhart L, Arndt C, Eikermann M. Incidence and duration of residual paralysis at the end of surgery after multiple administrations of cisatracurium and rocuronium. Anaesthesia. 2007;62:12–7.
Fuchs-Buder T, Schreiber JU, Meistelman C. Monitoring neuromuscular block: an update. Anaesthesia. 2009;64(Suppl 1):82–9.
Struys MM, De Smet T, Mortier EP. Simulated drug administration: an emerging tool for teaching clinical pharmacology during anesthesiology training. Clin Pharmacol Ther. 2008;84:170–4.
Wierda JM, Kleef UW, Lambalk LM, Kloppenburg WD, Agoston S. The pharmacodynamics and pharmacokinetics of Org 9426, a new non-depolarizing neuromuscular blocking agent, in patients anaesthetized with nitrous oxide, halothane and fentanyl. Can J Anaesth. 1991;38:430–5.
Szenohradszky J, Fisher DM, Segredo V, Caldwell JE, Bragg P, Sharma ML, Gruenke LD, Miller RD. Pharmacokinetics of rocuronium bromide (ORG 9426) in patients with normal renal function or patients undergoing cadaver renal transplantation. Anesthesiology. 1992;77:899–904.
Alvarez-Gomez JA, Estelles ME, Fabregat J, Perez F, Brugger AJ. Pharmacokinetics and pharmacodynamics of rocuronium bromide in adult patients. Eur J Anaesthesiol Suppl. 1994;9:53–6.
Cooper RA, Maddineni VR, Mirakhur RK, Wierda JM, Brady M, Fitzpatrick KT. Time course of neuromuscular effects and pharmacokinetics of rocuronium bromide (Org 9426) during isoflurane anaesthesia in patients with and without renal failure. Br J Anaesth. 1993;71:222–6.
Magorian T, Wood P, Caldwell J, Fisher D, Segredo V, Szenohradszky J, Sharma M, Gruenke L, Miller R. The pharmacokinetics and neuromuscular effects of rocuronium bromide in patients with liver disease. Anesth Analg. 1995;80:754–9.
Kleijn HJ, Zollinger DP, van den Heuvel MW, Kerbusch T. Population pharmacokinetic-pharmacodynamic analysis for sugammadex-mediated reversal of rocuronium-induced neuromuscular blockade. Br J Clin Pharmacol. 2011;72:415–33.
Kataria BK, Ved SA, Nicodemus HF, Hoy GR, Lea D, Dubois MY, Mandema JW, Shafer SL. The pharmacokinetics of propofol in children using three different data analysis approaches. Anesthesiology. 1994;80:104–22.
Murat I, Billard V, Vernois J, Zaouter M, Marsol P, Souron R, Farinotti R. Pharmacokinetics of propofol after a single dose in children aged 1–3 years with minor burns. Comparison of three data analysis approaches. Anesthesiology. 1996;84:526–32.
Egan TD, Lemmens HJ, Fiset P, Hermann DJ, Muir KT, Stanski DR, Shafer SL. The pharmacokinetics of the new short-acting opioid remifentanil (GI87084B) in healthy adult male volunteers. Anesthesiology. 1993;79:881–92.
Takagi S, Ozaki M, Iwasaki H, Hatano Y, Takeda J. [Effects of sevoflurane and propofol on neuromuscular blocking action of Org 9426 (rocuronium bromide) infused continuously in Japanese patients]. Masui. 2006;55:963–70.
Rex C, Wagner S, Spies C, Scholz J, Rietbergen H, Heeringa M, Wulf H. Reversal of neuromuscular blockade by sugammadex after continuous infusion of rocuronium in patients randomized to sevoflurane or propofol maintenance anesthesia. Anesthesiology. 2009;111:30–5.
Varvel JR, Donoho DL, Shafer SL. Measuring the predictive performance of computer-controlled infusion pumps. J Pharmacokinet Biopharm. 1992;20:63–94.
Glen JB, Servin F. Evaluation of the predictive performance of four pharmacokinetic models for propofol. Br J Anaesth. 2009;102:626–32.
Glass PS, Shafer S, Reves JG. Intravenous drug delivery systems. In: Miller RD, editor. Miller’s anesthesia. 6th ed. Pennsylvania: Elsevier; 2004. p. 439–80.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B. 1995;57:289–300.
Sepulveda P, Cortinez LI, Saez C, Penna A, Solari S, Guerra I, Absalom AR. Performance evaluation of paediatric propofol pharmacokinetic models in healthy young children. Br J Anaesth. 2011;107:593–600.
Masui K, Upton RN, Doufas AG, Coetzee JF, Kazama T, Mortier EP, Struys MM. The performance of compartmental and physiologically based recirculatory pharmacokinetic models for propofol: a comparison using bolus, continuous, and target-controlled infusion data. Anesth Analg. 2010;111:368–79.
Davidson JA, Macleod AD, Howie JC, White M, Kenny GN. Effective concentration 50 for propofol with and without 67% nitrous oxide. Acta Anaesthesiol Scand. 1993;37:458–64.
Miyabe-Nishiwaki T, Masui K, Kaneko A, Nishiwaki K, Nishio T, Kanazawa H. Evaluation of the predictive performance of a pharmacokinetic model for propofol in Japanese macaques (Macaca fuscata fuscata). J Vet Pharmacol Ther. 2013;36:169–73.
Olofsen E, Dinges DF, Van Dongen HP. Nonlinear mixed-effects modeling: individualization and prediction. Aviat Space Environ Med. 2004;75:A134–40.
Acknowledgments
The authors are grateful to MSD K.K. (Tokyo, Japan), a subsidiary of Merck & Co., Inc. (Whitehouse Station, NJ, USA), for providing the evaluation dataset of measured plasma concentrations of rocuronium.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sasakawa, T., Masui, K., Kazama, T. et al. The predictive ability of six pharmacokinetic models of rocuronium developed using a single bolus: evaluation with bolus and continuous infusion regimen. J Anesth 30, 620–627 (2016). https://doi.org/10.1007/s00540-016-2174-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-016-2174-5