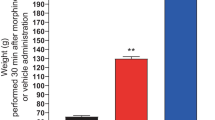
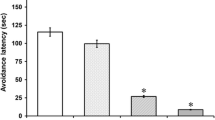
Abstract
Purpose
The N- and C-terminal regions of dynorphin (Dyn) A (1–17) activate opioid and N-methyl-D-aspartate receptors, respectively. Earlier studies demonstrated that Dyn-converting enzyme cleaved Dyn A (1–17) mainly at the Arg6–Arg7 bond, resulting in the production of N- and C-terminal region peptide fragments, and that this enzyme was not inhibited by a mixture of the three peptidase inhibitors (PIs) amastatin (A), captopril (C), and phosphoramidon (P). The purpose of the present study was to evaluate antinociceptive potential and toxicity with intracerebroventricular administration of Dyn A (1–17) or (1–13) under pretreatment with a mixture of A, C, and P and/or Dyn-converting enzyme inhibitor (p-hydroxymercuribenzoate).
Methods
Peptide fragments from Dyn A (1–17) following incubation with membrane preparation under pretreatment with a mixture of the three PIs was identified by matrix-assisted laser desorption ionization time-of-flight mass spectrometer (MALDI–TOF–MS). Infusion of drugs and peptides into the third ventricle in rats was performed via indwelling cannulae. Induction of antinociception and toxicity by Dyn A (1–17), Dyn A (1–13), Dyn A (1–6), or Dyn A (7–17) were determined by the tail-flick test and induction of barrel rotation, respectively. The effects of the PIs on antinociception and toxicity were evaluated by a dose−response study and a comparison of differences among various combinations of Dyn A (1–17) or Dyn A (1–13) and the three PIs and p-hydroxymercuribenzoate.
Results
MALDI–TOF–MS analysis identified Dyn A (1–6) and Dyn A (1–10) fragments as products following incubation of Dyn A (1–17) with membrane preparation of rat midbrain under pretreatment with a mixture of the three PIs. Pretreatment with a mixture of the three PIs produced an approximately 30-fold augmentation in antinociception induced by low-dose intracerebroventricular administration of Dyn A (1–17) or (1–13) in a μ-, δ- and κ-opioid receptor antagonist-reversible manner, but without signs of toxicity such as barrel rotation in the rat. Dyn A (1–17)-induced antinociception and toxicity was greater than that of Dyn A (1–6), Dyn A (1–13), or Dyn A (7–17) at the same dose. Dyn A (1–17)-induced antinociception and toxicity under pretreatment with various combinations of the three PIs and p-hydroxymercuribenzoate was greater than that with a mixture of the three PIs alone.
Conclusion
These findings suggest that administration of a mixture of the three PIs increases Dyn A (1–17)- or (1–13)-induced antinociception under physiological conditions without toxicity.










Similar content being viewed by others
References
Hauser KF, Aldrich JV, Anderson KJ, Bakalkin G, Christie MJ, Hall ED, Knapp PE, Scheff SW, Singh IN, Vissel B, Woods AS, Yakovleva T, Shippenberg TS. Pathobiology of dynorphins in trauma and disease. Front Biosci. 2005;10:216–35.
Silberring J, Castello ME, Nyberg F. Characterization of dynorphin A-converting enzyme in human spinal cord. An endoprotease related to a distinct conversion pathway for the opioid heptadecapeptide? J Biol Chem. 1992;267:21324–8.
Magnusson K, Hallberg M, Bergquist J, Nyberg F. Enzymatic conversion of dynorphin A in the rat brain is affected by administration of nandrolone decanoate. Peptides. 2007;28:851–8.
Oka T, Aoki K, Kajiwara M, Ishii K, Kuno Y, Hiranuma T, Matsumiya T. Inactivation of [Leu5]-enkephalin in three isolated preparations: relative importance of aminopeptidase, endopeptidase-24.11 and peptidyl dipeptidase A. NIDA Res Monogr. 1986;75:259–62.
Hiranuma T, Kitamura K, Taniguchi T, Kanai M, Arai Y, Iwao K, Oka T. Protection against dynorphin-(1-8) hydrolysis in membrane preparations by the combination of amastatin, captopril and phosphoramidon. J Pharmacol Exp Ther. 1998;286:863–9.
Akahori K, Kosaka K, Jin XL, Arai Y, Yoshikawa M, Kobayashi H, Oka T. Great increase in antinociceptive potency of [Leu5]enkephalin after peptidase inhibition. J Pharmacol Sci. 2008;106:295–300.
Kitamura K, Akahori K, Yano H, Iwao K, Oka T. Effects of peptidase inhibitors on anti-nociceptive action of dynorphin-(1-8) in rats. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:273–8.
Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–5.
Goldstein A, Fischli W, Lowney LI, Hunkapiller M, Hood L. Porcine pituitary dynorphin: complete amino acid sequence of the biologically active heptadecapeptide. Proc Natl Acad Sci USA. 1981;78:7219–23.
Hauser KF, Knapp PE, Turbek CS. Structure-activity analysis of dynorphin A toxicity in spinal cord neurons: intrinsic neurotoxicity of dynorphin A and its carboxyl-terminal, nonopioid metabolites. Exp Neurol. 2001;168:78–87.
Chavkin C, Goldstein A. Specific receptor for the opioid peptide dynorphin: structure–activity relationships. Proc Natl Acad Sci USA. 1981;78:6543–7.
Miura M, Yoshikawa M, Watanabe M, Takahashi S, Ajimi J, Ito K, Ito M, Kawaguchi M, Kobayashi H, Suzuki T. Increase in antinociceptive effect of [leu5]enkephalin after intrathecal administration of mixture of three peptidase inhibitors. Tokai J Exp Clin Med. 2013;38:62–70.
Bandler R, Carrive P, Depailis A. Emerging principles of organization of the midbrain periaqueductal gray matter. New York: Plenum Press; 1991.
Hiranuma T, Oka T. Effects of peptidase inhibitors on the [Met5]-enkephalin hydrolysis in ileal and striatal preparations of guinea-pig: almost complete protection of degradation by the combination of amastatin, captopril and thiorphan. Jpn J Pharmacol. 1986;41:437–46.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates; 1986.
Janssen PA, Niemegeers CJ, Dony JG. The inhibitory effect of fentanyl and other morphine-like analgesics on the warm water induced tail withdrawl reflex in rats. Arzneimittelforschung. 1963;13:502–7.
Yeomans L, Muthu D, Lowery JJ, Martinez HN, Abrell L, Lin G, Strom K, Knapp BI, Bidlack JM, Bilsky EJ, Polt R. Phosphorylation of enkephalins: NMR and CD studies in aqueous and membrane-mimicking environments. Chem Biol Drug Des. 2011;78:749–56.
Walker EA. In vivo pharmacological resultant analysis reveals non-competitive interactions between opioid antagonists in the rat tail-withdrawal assay. Br J Pharmacol. 2006;149:1071–82.
Xie H, Woods JH, Traynor JR, Ko MC. The spinal antinociceptive effects of endomorphins in rats: behavioral and G protein functional studies. Anesth Analg. 2008;106:1873–81.
Malmberg AB, Yaksh TL. Isobolographic and dose-response analyses of the interaction between intrathecal mu and delta agonists: effects of naltrindole and its benzofuran analog (NTB). J Pharmacol Exp Ther. 1992;263:264–75.
Kaneko T, Nakazawa T, Ikeda M, Yamatsu K, Iwama T, Wada T, Satoh M, Takagi H. Sites of analgesic action of dynorphin. Life Sci. 1983;33(Suppl 1):661–4.
Martinka GP, Jhamandas K, Sabourin L, Lapierre C, Lemaire S. Dynorphin A-(1–13)-Tyr14-Leu15-Phe16-Asn17-Gly18-Pro19: a potent and selective kappa opioid peptide. Eur J Pharmacol. 1991;196:161–7.
Voorn P, van de Witte SV, Li K, Jonker AJ. Dynorphin displaces binding at the glycine site of the NMDA receptor in the rat striatum. Neurosci Lett. 2007;415:55–8.
Reed B, Zhang Y, Chait BT, Kreek MJ. Dynorphin A(1-17) biotransformation in striatum of freely moving rats using microdialysis and matrix-assisted laser desorption/ionization mass spectrometry. J Neurochem. 2003;86:815–23.
Tan-No K, Ohshima K, Taira A, Inoue M, Niijima F, Nakagawasai O, Tadano T, Nylander I, Silberring J, Terenius L, Kisara K. Antinociceptive effect produced by intracerebroventricularly administered dynorphin A is potentiated by p-hydroxymercuribenzoate or phosphoramidon in the mouse formalin test. Brain Res. 2001;891:274–80.
Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain. 1996;68:141–50.
Lu Y, Sweitzer SM, Laurito CE, Yeomans DC. Differential opioid inhibition of C- and A delta-fiber mediated thermonociception after stimulation of the nucleus raphe magnus. Anesth Analg. 2004;98:414–9 table of contents.
Morgan MM, Fossum EN, Stalding BM, King MM. Morphine antinociceptive potency on chemical, mechanical, and thermal nociceptive tests in the rat. J Pain. 2006;7:358–66.
Mansour A, Hoversten MT, Taylor LP, Watson SJ, Akil H. The cloned mu, delta and kappa receptors and their endogenous ligands: evidence for two opioid peptide recognition cores. Brain Res. 1995;700:89–98.
Naqvi T, Haq W, Mathur KB. Structure-activity relationship studies of dynorphin A and related peptides. Peptides. 1998;19:1277–92.
Pan ZZ. mu-Opposing actions of the kappa-opioid receptor. Trends Pharmacol Sci. 1998;19:94–8.
Acknowledgments
The authors would like to thank Professor Jeremy Williams, Tokyo Medical University, for his assistance with the English of the manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ajimi, J., Yoshikawa, M., Takahashi, S. et al. Effect of three peptidase inhibitors on antinociceptive potential and toxicity with intracerebroventricular administration of dynorphin A (1–17) or (1–13) in the rat. J Anesth 29, 65–77 (2015). https://doi.org/10.1007/s00540-014-1860-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-014-1860-4