Abstract
Background
Lipopolysaccharide (LPS) induces visceral hypersensitivity, and corticotropin-releasing factor (CRF) also modulates visceral sensation. Besides, LPS increases CRF immunoreactivity in rat colon, which raises the possibility of the existence of a link between LPS and the CRF system in modulating visceral sensation. The present study tried to clarify this possibility.
Methods
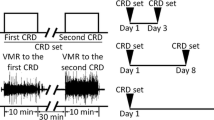
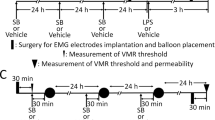
Visceral sensation was assessed by abdominal muscle contractions induced by colonic balloon distention, i.e., visceromotor response, electrophysiologically in conscious rats. The threshold of visceromotor response was measured before and after administration of drugs.
Results
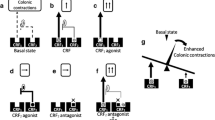
LPS at a dose of 1 mg/kg subcutaneously (sc) decreased the threshold at 3 h after the administration. Intraperitoneal (ip) administration of anakinra (20 mg/kg), an interleukin-1 (IL-1) receptor antagonist, or interleukin-6 (IL-6) antibody (16.6 µg/kg) blocked this effect. Additionally, IL-1β (10 µg/kg, sc) or IL-6 (10 µg/kg, sc) induced visceral allodynia. Astressin (200 µg/kg, ip), a non-selective CRF receptor antagonist, abolished the effect of LPS, but astressin2-B (200 µg/kg, ip), a CRF receptor type 2 (CRF2) antagonist, did not alter it. Peripheral CRF receptor type 1 (CRF1) stimulation by cortagine (60 µg/kg, ip) exaggerated the effect of LPS, but activation of CRF2 by urocortin 2 (60 µg/kg, ip) abolished it.
Conclusions
LPS induced visceral allodynia possibly through stimulating IL-1 and IL-6 release. In addition, this effect was mediated through peripheral CRF signaling. Since the LPS–cytokine system is thought to contribute to altered visceral sensation in the patients with irritable bowel syndrome, these results may further suggest that CRF plays a crucial role in the pathophysiology of this disease.






Similar content being viewed by others
References
Taché Y, Kiank C, Stengel A. A role for corticotropin-releasing factor in functional gastrointestinal disorders. Curr Gastroenterol Rep. 2009;11:270–7.
Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–86.
Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann N Y Acad Sci. 1999;885:312–28.
Martínez V, Wang L, Million M, et al. Urocortins and the regulation of gastrointestinal motor function and visceral pain. Peptides. 2004;25:1733–44.
Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front Neuroendocrinol. 2007;28:1–27.
Talley NJ, Spiller R. Irritable bowel syndrome: a little understood organic bowel disease? Lancet. 2002;360:555–64.
Nozu T, Okumura T. Corticotropin-releasing factor receptor type 1 and type 2 interaction in irritable bowel syndrome. J Gastroenterol. 2015;50:819–30.
Elsenbruch S. Abdominal pain in irritable bowel syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun. 2011;25:386–94.
Bercik P, Verdu EF, Collins SM. Is irritable bowel syndrome a low-grade inflammatory bowel disease? Gastroenterol Clin N Am. 2005;34:235–45.
Barbara G, Zecchi L, Barbaro R, et al. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J Clin Gastroenterol. 2012;46(Suppl):S52–5.
Moriez R, Salvador-Cartier C, Theodorou V, et al. Myosin light chain kinase is involved in lipopolysaccharide-induced disruption of colonic epithelial barrier and bacterial translocation in rats. Am J Pathol. 2005;167:1071–9.
Dlugosz A, Nowak P, D’Amato M, et al. Increased serum levels of lipopolysaccharide and antiflagellin antibodies in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2015;27:1747–54.
Coelho AM, Fioramonti J, Bueno L. Systemic lipopolysaccharide influences rectal sensitivity in rats: role of mast cells, cytokines, and vagus nerve. Am J Physiol Gastrointest Liver Physiol. 2000;279:G781–90.
Benson S, Kattoor J, Wegner A, et al. Acute experimental endotoxemia induces visceral hypersensitivity and altered pain evaluation in healthy humans. Pain. 2012;153:794–9.
Yuan PQ, Wu SV, Wang L, et al. Corticotropin releasing factor in the rat colon: expression, localization and upregulation by endotoxin. Peptides. 2010;31:322–31.
Yuan PQ, Wu SV, Pothoulakis C, et al. Urocortins and CRF receptor type 2 variants in the male rat colon: gene expression and regulation by endotoxin and anti-inflammatory effect. Am J Physiol Gastrointest Liver Physiol. 2016;. doi:10.1152/ajpgi.00337.2015.
Tsuchiya Y, Nozu T, Kumei S, et al. IL-1 receptor antagonist blocks the lipopolysaccharide-induced inhibition of gastric motility in freely moving conscious rats. Dig Dis Sci. 2012;57:2555–61.
Nozu T, Takakusaki K, Okumura T. A balance theory of peripheral corticotropin-releasing factor receptor type 1 and type 2 signaling to induce colonic contractions and visceral hyperalgesia in rats. Endocrinology. 2014;155:4655–64.
Nozu T, Kumei S, Miyagishi S, et al. Colorectal distention induces acute and delayed visceral hypersensitivity: role of peripheral corticotropin-releasing factor and interleukin-1 in rats. J Gastroenterol. 2015;50:1153–61.
Tuna M, Erman T, Ildan F, et al. Effect of neutralization of rat IL-6 bioactivity on collateral blood supply from retrograde flow via cortical anastomoses in the rat central nervous system. Neurol Res. 2002;24:405–8.
Toth B, Yokoyama Y, Schwacha MG, et al. Insights into the role of interleukin-6 in the induction of hepatic injury after trauma-hemorrhagic shock. J Appl Physiol. 1985;2004(97):2184–9.
Million M, Wang L, Wang Y, et al. CRF2 receptor activation prevents colorectal distension induced visceral pain and spinal ERK1/2 phosphorylation in rats. Gut. 2006;55:172–81.
Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153–69.
Okumura T, Nozu T, Kumei S, et al. Antinociceptive action against colonic distension by brain orexin in conscious rats. Brain Res. 2015;1598:12–7.
Nozu T, Miyagishi S, Nozu R, et al. Water avoidance stress induces visceral hyposensitivity through peripheral corticotropin releasing factor receptor type 2 and central dopamine D2 receptor in rats. Neurogastroenterol Motil. 2015. doi:10.1111/nmo.12747.
Tang QL, Lai ML, Zhong YF, et al. Antinociceptive effect of berberine on visceral hypersensitivity in rats. World J Gastroenterol. 2013;19:4582–9.
Coelho A, Fioramonti J, Bueno L. Brain interleukin-1beta and tumor necrosis factor-alpha are involved in lipopolysaccharide-induced delayed rectal allodynia in awake rats. Brain Res Bull. 2000;52:223–8.
Obreja O, Rathee PK, Lips KS, et al. IL-1 beta potentiates heat-activated currents in rat sensory neurons: involvement of IL-1RI, tyrosine kinase, and protein kinase C. FASEB J. 2002;16:1497–503.
Schafers M, Sorkin LS, Geis C, et al. Spinal nerve ligation induces transient upregulation of tumor necrosis factor receptors 1 and 2 in injured and adjacent uninjured dorsal root ganglia in the rat. Neurosci Lett. 2003;347:179–82.
Leo M, Argalski S, Schafers M, et al. Modulation of voltage-gated sodium channels by activation of tumor necrosis factor receptor-1 and receptor-2 in small DRG neurons of rats. Mediat Inflamm. 2015. doi:10.1155/2015/124942.
Cunha TM, Verri WA Jr, Silva JS, et al. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci. 2005;102:1755–60.
Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819.
von Banchet GS, Kiehl M, Schaible HG. Acute and long-term effects of IL-6 on cultured dorsal root ganglion neurones from adult rat. J Neurochem. 2005;94:238–48.
Brenn D, Richter F, Schaible HG. Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: an inflammatory mechanism of joint pain. Arthritis Rheum. 2007;56:351–9.
Schaible HG. Nociceptive neurons detect cytokines in arthritis. Arthritis Res Ther. 2014;16:470.
Buckley MM, O’Halloran KD, Rae MG, et al. Modulation of enteric neurons by interleukin-6 and corticotropin-releasing factor contributes to visceral hypersensitivity and altered colonic motility in a rat model of irritable bowel syndrome. J Physiol. 2014;592:5235–50.
Maier SF, Watkins LR. Immune-to-central nervous system communication and its role in modulating pain and cognition: implications for cancer and cancer treatment. Brain Behav Immun. 2003;17(Suppl 1):S125–31.
Laye S, Parnet P, Goujon E, et al. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res Mol Brain Res. 1994;27:157–62.
Larauche M, Kiank C, Taché Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J Physiol Pharmacol. 2009;60(Suppl 7):33–46.
Gourcerol G, Wu SV, Yuan PQ, et al. Activation of corticotropin-releasing factor receptor 2 mediates the colonic motor coping response to acute stress in rodents. Gastroenterology. 2011;140(1586–96):e6.
Million M, Maillot C, Adelson DA, et al. Peripheral injection of sauvagine prevents repeated colorectal distension-induced visceral pain in female rats. Peptides. 2005;26:1188–95.
Yuan PQ, Wu SV, Taché Y. Urocortins and CRF type 2 receptor isoforms expression in the rat stomach are regulated by endotoxin: role in the modulation of delayed gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2012;303:G20–31.
O’malley D, Julio-Pieper M, Gibney SM, et al. Differential stress-induced alterations of colonic corticotropin-releasing factor receptors in the Wistar Kyoto rat. Neurogastroenterol Motil. 2010;22:301–11.
Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014;13:924–35.
Larauche M, Gourcerol G, Wang L, et al. Cortagine, a CRF1 agonist, induces stresslike alterations of colonic function and visceral hypersensitivity in rodents primarily through peripheral pathways. Am J Physiol Gastrointest Liver Physiol. 2009;297:G215–27.
Agelaki S, Tsatsanis C, Gravanis A, et al. Corticotropin-releasing hormone augments proinflammatory cytokine production from macrophages in vitro and in lipopolysaccharide-induced endotoxin shock in mice. Infect Immun. 2002;70:6068–74.
Heinrichs SC, De Souza EB, Schulteis G, et al. Brain penetrance, receptor occupancy and antistress in vivo efficacy of a small molecule corticotropin releasing factor type I receptor selective antagonist. Neuropsychopharmacology. 2002;27:194–202.
Rorato R, Reis WL, de Carvalho Borges B, et al. Cannabinoid CB1 receptor restrains accentuated activity of hypothalamic corticotropin-releasing factor and brainstem tyrosine hydroxylase neurons in endotoxemia-induced hypophagia in rats. Neuropharmacology. 2012;63:154–60.
Ortiz-Lucas M, Saz-Peiro P, Sebastian-Domingo JJ. Irritable bowel syndrome immune hypothesis. Part two: the role of cytokines. Rev Esp Enferm Dig. 2010;102:711–7.
Bruewer M, Luegering A, Kucharzik T, et al. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–72.
Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–20.
Acknowledgments
This work was supported in part by grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan [C-26460287 (TN) and C-26460955 (TO)].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nozu, T., Miyagishi, S., Nozu, R. et al. Lipopolysaccharide induces visceral hypersensitivity: role of interleukin-1, interleukin-6, and peripheral corticotropin-releasing factor in rats. J Gastroenterol 52, 72–80 (2017). https://doi.org/10.1007/s00535-016-1208-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-016-1208-y