Abstract
Background
Long-term nucleos(t)ide analogue (NA) therapy for chronic hepatitis B (CHB) patients has been reported to reduce the risk of hepatocellular carcinoma (HCC) development. However, survival rates and causes of death in CHB patients either treated or not treated with NA therapy are unclear. Therefore, we investigated the prognosis of CHB in both of these groups.
Methods
A total of 919 CHB patients who were treated (n = 189) or not treated (n = 730) with NA therapy were enrolled; of these, 135 were selected from each group by propensity score matching. Survival, mortality from both HCC and non-liver related diseases, and causes of death were analyzed.
Results
In all patients (n = 919), cumulative survival and mortality from both HCC and non-liver related diseases did not differ significantly according to NA therapy status. Of 66 patients who died during the follow-up period, 59.1 % died due to liver-related diseases (including HCC); of the remainder, 48.1 % died of non-liver related malignancies. In patients selected by propensity score matching (n = 270), cumulative survival and mortality from HCC were significantly improved in those who received NA therapy compared with those who did not (p = 0.015 and 0.018, respectively). Cox proportional hazards models indicated that NA therapy was independently associated with survival of CHB patients (hazard ratio, 0.286; 95 % confidence interval, 0.122–0.668; p = 0.004).
Conclusions
Approximately 40 % of CHB patients died of non-liver-related diseases. Additionally, in patients who required anti-viral therapy for CHB, NA therapy improved survival and mortality from HCC.
Similar content being viewed by others
Introduction
Chronic hepatitis B (CHB) affects over 350 million people worldwide. Long-term complications of infection include cirrhosis and hepatocellular carcinoma (HCC), which together cause over 500,000 deaths annually [1, 2]. Hepatitis B surface antigen (HBsAg)-positive patients have a 70-fold increased risk of developing HCC compared to their HBsAg-seronegative counterparts [3, 4]. Hepatitis B virus (HBV) infection is endemic in Southeast Asia, China, Taiwan, Korea, and sub-Saharan Africa, where up to 85–95 % of patients with HCC are HBsAg positive [5]. HCC is the third and fifth leading cause of cancer deaths in men and women, respectively, and the number of deaths and the mortality rate from HCC have greatly increased in Japan since 1975 [6]. Hepatitis C virus–related HCC accounts for 75 % of all HCC in Japan, while HBV-related HCC accounts for 15 % [6].
Nucleos(t)ide analogues (NAs) are an established treatment for CHB [7–9]. Between 2000 and 2006, lamivudine, adefovir dipivoxil, and entecavir were approved in Japan as NA therapies for CHB, and in 2014 tenofovir disoproxil fumarate was also approved. NAs have a powerful inhibitory effect on HBV DNA proliferation, regardless of genotype, and act as antiviral agents and promote quiescence of hepatitis in nearly all patients, including those of more advanced age with little prospect of spontaneous remission. NA therapy for CHB has been reported to not only prevent the progression of hepatitis, but to also reduce the risk of development of HCC [10, 11]. However, survival rates and causes of death, including those that are non-liver-related, have not been sufficiently investigated in CHB patients receiving or not receiving long-term NA therapy.
In the present study, we clarified these issues and also confirmed the impact of NA therapy on decreasing mortality in patients with CHB, using propensity score analysis to reduce biases associated with the selection of study patients [12–15].
Materials and methods
Patients
The study protocol was approved by the Institutional Ethics Committee of Ogaki Municipal Hospital in January 2011, and was in compliance with the Declaration of Helsinki. Written informed consent for the use of stored serum samples was obtained from all patients.
Between 1991 and 2010, 2220 consecutive HBsAg-positive patients who visited the Department of Gastroenterology and Hepatology at Ogaki Municipal Hospital were prospectively enrolled in our HCC surveillance program. These patients included 1220 patients (55.0 %) in whom hepatocarcinogenesis was investigated in our previous study [11]. Of these, 919 met the following inclusion criteria: HBsAg-positive for more than 6 months; no evidence of HCV co-infection; no other causes of chronic liver disease (alcohol consumption >80 g/day, hepatotoxic drugs, autoimmune hepatitis, primary biliary cirrhosis, hemochromatosis, and Wilson’s disease); no incomplete clinical data or missing serum samples; follow-up duration of greater than 3 years; no evidence of malignancies, including HCC, for at least 1 year from the start of the follow-up period; and receiving NA therapy for more than 1 year before the detection of HCC. In patients on NA therapy, the date of NA therapy initiation was considered the start of the follow-up period. In the non-NA group (controls), the date of the first visit was defined as the start of follow-up. The end of follow-up was defined as the final visit for patients who had not died, and as the date of death for patients who died during follow-up.
Of the 919 eligible patients, 189 received NA therapy during the follow-up period (NA group) and 730 patients did not (non-NA group). We first compared the survival rates between the two groups and determined the causes of death in all patients. Then, to reduce the confounding effects of covariates, we used propensity scores to match NA patients to unique non-NA patients. Eight covariates, including age, sex, HBV DNA concentration, HBsAg, hepatitis B e antigen (HBeAg), genotype, platelet count, and alanine aminotransferase (ALT) activity were taken into account at the start of follow-up. Based on previously reported cut-off values for NA therapy indications or relation to the progression of HBV patients [16–19], we computed the propensity scores using logistic regression with the following independent variables: age (≤40 years or >40 years), sex (female or male), HBV DNA concentration (≤5.0 log copies/ml or >5.0 log copies/ml), HBsAg concentration (≤3.0 log copies/ml or >3.0 log copies/ml), HBeAg (negative or positive), genotype (genotype C or non-genotype C), platelet count (>150 × 103/m3 or ≤150 × 103/m3), and ALT activity (≤35 IU/ml or >35 IU/ml). The calculated propensity scores of the NA and non-NA groups were 0.22079–0.98208 (median, 0.5072) and 0.22079–0.99659 (median, 0.9206), respectively; these scores were then rounded to two decimal places. We conducted one-to-one matching of patients based on consistency of propensity scores to the second decimal place. Propensity score matching resulted in the selection of 270 patients (NA group, 135 patients; non-NA group, 135 patients) (Fig. 1). The p value of the calculated propensity score was 0.372 based on the Hosmer–Lemeshow test [20]. The area under the curve (AUC) of the receiver operating characteristic (ROC)-calculated propensity score was 0.862 [95 % confidence interval (CI), 0.834–0.891] [21].
Surveillance, diagnosis, and causes of death
All patients were followed up at our hospital at least every 6 months. During each follow-up examination, we measured platelet counts and levels of ALT, gamma-glutamyl transpeptidase (γ-GTP), total bilirubin, alkaline phosphatase (ALP), albumin, and alphafetoprotein (AFP). We used commercially available kits to test blood samples for HBsAg, HBeAg, and anti-HBe (Abbott Japan, Tokyo, Japan). After December 2007, which was the start of the follow-up period for the CHB patients, serum HBV DNA concentrations were monitored by polymerase chain reaction assay (COBAS AmpliPrep-COBAS TaqMan HBV Test v2.0, Roche Diagnostics), with a lower detection limit of approximately 2.1 log copies/ml. Before November 2007, these concentrations were measured once at the start of the follow-up period using patients’ stored frozen serum (80 °C) with the COBAS TaqMan HBV Test v2.0. HBV genotyping was performed as previously described [22]. Serum levels of HBV core-related antigen (HBcrAg) were measured using a chemiluminescence enzyme immunoassay (CLEIA) as previously described [23, 24]. Precore nucleotide 1896 and basal core promoter (BCP) dinucleotide 1762/1764 were determined using the line probe assay (INNO-LiPA HBV PreCore assay; Innogenetics NV) [25, 26]. The probes were designed to determine the nucleotides at position 1896 (G vs. A) in the precore region and positions 1762 (A vs. T) and 1764 (G vs. A and G vs. T) in the BCP region. A line probe assay was used to identify any emergence of YMDD mutations (INNO-LiPA HBV DR assay; Innogenetics NV).
In accordance with the Clinical Practice Guidelines for Hepatocellular Carcinoma in Japan [27], cirrhotic patients under surveillance underwent ultrasound (US) and monitoring of tumor markers every 3–4 months, and dynamic computed tomography (CT) or magnetic resonance imaging (MRI) every 12 months. For patients with chronic hepatitis, we performed US and monitoring of tumor markers every 6 months. The diagnosis of cirrhosis was made based on histological examination or typical US findings, e.g., superficial nodularity, a coarse parenchymal echo pattern, and signs of portal hypertension (splenomegaly >120 mm, dilated portal vein diameter >12 mm, patent collateral veins, or ascites) [28–30]. Patients who did not satisfy these criteria were classified as having chronic hepatitis. As recommended by the diagnosis algorithm of the Japan Society of Hepatology [27], HCC was diagnosed principally based on the results from ultrasonography and dynamic CT (hyperattenuation during the arterial phase in all or part of the tumor, and hypoattenuation in the portal venous phase) and/or MRI.
Diseases other than HCC were initially detected based on clinical symptoms and/or abnormal surveillance data, medical check-ups (in community or workplace), or assessment of physicians. These conditions were then diagnosed based on disease-specific criteria by the appropriate specialists in our hospital. Causes of death data were defined by these specialists using the International Statistical Classification of Diseases and Related Health Problems (ICD) codes (ICD-9 codes for deaths occurring prior to 1 January 2003, and thereafter ICD-10 codes) [31]. All of the studies were performed retrospectively by collecting and analyzing data from the patient records.
Ogaki Municipal Hospital is located in a region of 400,000 inhabitants and is the only general hospital in the region employing ten or more gastroenterologists. Therefore, a large number of CHB patients requiring HCC surveillance visit regularly as outpatients. Additionally, there is close contact, including sharing of patient mortality data (if the patients died other than in our hospital), between family physician clinic or care hospital in community and our hospital.
Treatments
The 135 patients in the NA group received the following NA therapies: lamivudine (17 patients), lamivudine and adefovir dipivoxil (26 patients), and entecavir (92 patients). The indications for NA therapy in each patient were determined according to the guidelines of the American Association for the Study of Liver Diseases (AASLD), the European Association for the Study of the Liver (EASL), or the Asian Pacific Association for the Study of the Liver (APASL) [7–9]. Of the 135 patients in the non-NA group, 103 did not receive treatment because at the time of their enrollment NA had not yet been approved in Japan, while the remaining 32 patients declined NA therapy.
Statistical analysis
Continuous variables are expressed as medians (range). The Mann–Whitney U test was used for continuous variables, and the Chi square test with Yates’ correction or Fisher’s exact test was used for categorical variables. Actuarial analysis of cumulative survival and mortality was performed using the Kaplan–Meier method, and differences were tested with the log-rank test. Cox proportional hazards models with forward selection were used for multivariate analysis of factors related to survival.
Discrimination of the propensity score model was assessed using the area under the ROC curve [21], with higher values indicating better discrimination. Calibration was assessed using the Hosmer–Lemeshow goodness-of-fit test [20]. The Hosmer–Lemeshow test compares model performance (observed versus expected) across deciles of risk to test whether the model is biased (i.e., performs differently at the extremes of risk). A non-significant value for the Hosmer–Lemeshow test suggests an absence of such bias.
We considered p values of 0.05 or less to be significant. Statistical analysis was performed with SPSS, version 18.0 for Windows (IBM Japan, Tokyo, Japan).
Results
Patient characteristics and causes of death in all patients
Table 1 shows baseline characteristics of all 919 patients before propensity matching. There were significant differences in age, HBV genotype, HBsAg concentration, HBV DNA concentration, HBcrAg concentration, presence of HBeAg, BCP mutations, platelet count, ALT level, γ-GTP level, and history of interferon (IFN) therapy. HCC developed in 24 of 189 patients (12.7 %) in the NA group and 66 of 730 patients (9.0 %) in the non-NA-group during the follow-up period, respectively. Initial treatments of the HCCs are also shown in Table 1. Of the 919 patients, 66 died during follow-up; causes of death are shown in Table 2. Mortality was due to liver-related diseases in 59.1 % (39/66) of patients, with HCC responsible in 82.1 % (32/39) of these. Conversely, in 48.1 % (13/27) of patients who died of non-liver-related diseases, the causes of death were a variety of malignancies other than HCC, including hematological diseases. In non-liver-related diseases other than malignancies, the feature of the causes of death was also not found. There were no significant differences between NA and non-NA groups in terms of causes of death, whether liver-related or non-liver-related.
Patient characteristics and causes of death determined after propensity score matching
The baseline characteristics of the 270 study patients after propensity score matching are summarized in Table 3. There were no significant differences in age, sex, HBV genotype, HBsAg concentration, HBV DNA concentration, HBcrAg concentration, presence of HBeAg, precore region mutations, BCP mutations, platelet count, ALT level, γ-GTP level, history of IFN therapy, or follow-up duration. NA was administered for a median of 5.5 years (range 1.0–10.0 years). HCC developed in 19 of 135 patients (14.1 %) in the NA group and 37 of 135 patients (27.4 %) in the non-NA-group during the follow-up period, respectively. Initial treatments of the HCCs are also shown in Table 3. In the NA group, eight of the 135 patients died during follow-up, and of these 62.5 % (5/8) died due to HCC. Conversely, 23 of 135 patients in the non-NA group died during follow-up, and of these 73.9 % (17/23) patients died due to HCC. Only three patients (13.0 %) died due to malignancies other than HCC.
Cumulative survival and mortality analysis
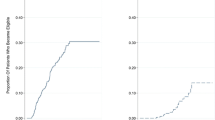
Figure 2a shows the survival curves for all 919 patients. The respective 5-, 10-, and 15-year cumulative survival rates were 97.7, 94.6, and 91.0 % in the NA-group patients (n = 189), and 99.6, 94.2, and 89.1 % in the non-NA-group patients (n = 730) (p = 0.868). In the survival analysis of the absence or presence of cirrhosis, there were no differences between the NA-group and the non-NA-group in the status of cirrhosis. Additionally, the respective 5-, 10-, and 15-year cumulative mortality rates from HCC were 0.6, 3.1, and 4.7 % in NA-group patients, and 0.2, 1.0, and 5.5 % in non-NA-group patients (p = 0.788) (Fig. 2b). In the mortality from HCC analysis of the absence or presence of cirrhosis, there was no difference between the NA-group and the non-NA-group in the non-cirrhotic patients. Conversely, in the cirrhotic patients, the respective 5-, 10-, and 15-year cumulative mortality rates from HCC were 1.5, 5.3, and 5.3 % in the NA- group (n = 70), and 1.2, 8.7, and 18.5 % in the non-NA-group (n = 91) (p = 0.047).
a Cumulative survival in all chronic hepatitis B (CHB) patients (before propensity score matching) according to nucleos(t)ide analogue (NA) treatment status. b Cumulative mortality from hepatocellular carcinoma (HCC) in all CHB patients (before propensity score matching) according to NA treatment status. There are no significant differences between NA and non-NA groups in either cumulative survival or mortality from HCC
Figure 3a shows the survival curves for 270 patients after propensity score matching. The respective 5, 10, and 15-year cumulative survival rates were 99.2, 94.8, and 91.3 % in NA-group patients (n = 135), and 100, 89.4, and 75.4 % in non-NA-group patients (n = 135) (p = 0.015). In the survival analysis of the absence or presence of cirrhosis, there were no differences between the NA-group and the non-NA-group in the status of cirrhosis. Additionally, the respective 5, 10, and 15-year cumulative mortality rates from HCC were 0.0, 3.5, and 5.8 % in NA-group patients, and 0.0, 7.1, and 19.2 % in non-NA-group patients, (p = 0.018) (Fig. 3b). In the mortality from HCC analysis of the absence or presence of cirrhosis, there was no difference between the NA-group and the non-NA-group in the non-cirrhotic patients. Conversely, in the cirrhotic patients, the respective 5, 10, and 15-year cumulative mortality rates from HCC were 0.0, 5.2, and 5.2 % in the NA- group (n = 51), and 0.0, 11.2, and 30.3 % in the non-NA-group (n = 41) (p = 0.017). In the survival and mortality from HCC analysis of three types of NAs therapies, there were no differences among them.
a Cumulative survival in chronic hepatitis B (CHB) patients (after propensity score matching) according to nucleos(t)ide analogue (NA) treatment status. b Cumulative mortality from hepatocellular carcinoma (HCC) in all CHB patients (after propensity score matching) according to NA treatment status. There are significant differences between NA and non-NA groups in both cumulative survival (p = 0.015) and mortality from HCC (p = 0.018)
Figure 4 shows the cumulative mortality from non-liver-related diseases. There were no significant differences between the NA and non-NA groups (a, all 919 patients; b, 270 propensity score–matched patients).
Factors associated with patient survival determined after propensity score matching
Multivariate analysis with Cox proportional hazards modeling using the covariates of age (≤40 years or >40 years), sex (female or male), treatment (NA or non-NA), HBsAg (≤ 3.0 log IU/ml or >3.0 log IU/ml), HBV DNA level (≤5.0 log copies/ml or >5.0 log copies/ml), HBeAg (negative or positive), precore region (wild type or mutant), BCP (wild type or mutant type), HBcrAg (≤ 3.0 log U/ml or > 3.0 log U/ml), genotype (genotype C or non-genotype C), platelet count (>150 × 103/m3 or ≤150 × 103/m3), ALT (≤35 IU/ml or >35 IU/ml), and γ-GTP (≤56 IU/ml or >56 IU/ml) showed that NA therapy was an independent factor associated with improved patient survival (hazard ratio [HR], 0.286; 95 % confidence interval [CI], 0.122–0.668; p = 0.004).
Discussion
In the present study, which used propensity score analysis to reduce biases associated with the selection of study patients, long-term NA therapy significantly reduced the cumulative mortality from HCC in CHB patients. In addition, there was no significant difference in non-liver-related mortality between the NA and non-NA groups. These results demonstrated that NA therapy improved the survival of patients who required anti-viral therapy for CHB. Moreover, multivariate analysis with Cox proportional hazards models showed that NA therapy was an independent factor associated with improved survival of CHB patients.
We recently reported that NA therapy reduced the risk of HCC in patients with CHB [11]. In that study, which also used propensity score analysis, the respective 5-, 7-, and 10-year cumulative incidences of HCC were 2.7, 3.3, and 3.3 % in patients on NA therapy (n = 117) and 11.3, 26.0, and 40.0 % in patients not on NA therapy (n = 117). Further, multivariate analysis with Cox proportional hazards models showed that NA therapy significantly reduced the risk of hepatocarcinogenesis in CHB patients (HR, 0.28; 95 % CI, 0.13–0.62). In the present study, we further assessed the survival and the mortality from both HCC and non-liver related diseases, expanding the number of study patients compared with our previous study for hepatocarcinogenesis. The present study, which demonstrated improved survival of CHB patients on NA therapy, supports our previous results that showed a reduction in hepatocarcinogenesis by NAs. Conversely, other factors that were associated with the development of HCC in that study, including higher age, BCP mutations, and high HBcrAg and γ-GTP concentrations, were not identified in this study as independent factors influencing survival of CHB patients. It was considered that these factors associated with hepatocarcinogenesis [11] did not influence the survival of CHB patients, especially, after HCC development. NA therapy for CHB patients has been reported to not only prevent disease progression from advanced liver disease but also to reverse decompensated cirrhosis [32–34]. Thus even if HCC has developed in patients receiving NA, it is assumed that treatment of recurrent HCC is possible while maintaining liver function. In the present study, particularly, in the analysis of cirrhotic patients, the cumulative mortality rates from HCC in the NA-group were significantly lower than in the non-NA-group in both all and propensity score matched patients.
Chen et al. [35] used community cohort data to analyze mortality from non-liver-related causes of death in patients with CHB. They reported that the relative risks (RRs) and 95 % CIs for all non-liver-related deaths among HBsAg-positive subjects were 1.2 (1.1–1.3) in males and 1.4 (1.1–1.7) in females. Non-liver-related causes were further subdivided into cancer and non-cancer groups. For all non-liver cancers, the RRs were 1.2 (1.0–1.4) for males and 1.7 (1.2–2.3) for females. Non-cancer deaths that were non-liver-related had RRs of 1.2 (1.1–1.4) and 1.2 (0.9–1.6) in males and females, respectively. They concluded that HBV-infected individuals may be at increased mortality risk from non-liver-related causes; possible reasons include the direct effect of HBV infection, changes in the host immune system as a cause or effect of chronic infection, and behavioral factors associated with HBV infection.
In the present study, 66 of all 919 CHB patients died during follow-up; in approximately 40 % (27/66) of cases the causes of death were non-liver-related diseases, of which about 50 % (13/27) were malignancies other than HCC. Although this study was based on hospital-based subjects, we performed detailed analysis to categorize NA administration status in CHB patients compared with Chen et al.’s study. Additionally, our study revealed no significant difference in cumulative mortality between the NA and non-NA groups before and after propensity score matching. Further, malignancies arose from a variety of organs, and thus we recommend that CHB patients be monitored not only for the development of liver-related diseases but non-liver-related disease as well, particularly malignancies.
Since the present study was retrospective in nature, we used propensity score analysis to reduce the selection bias associated with indications for NA therapy. The p value of 0.372 by the Hosmer–Lemeshow test, which evaluates the goodness-of-fit for the calculated propensity score, was considered reassuring [20]. Additionally, the AUC of 0.862 (95 % CI, 0.834–0.891) in the ROC analysis suggested excellent discrimination for the calculated propensity score [21]. Consequently, the backgrounds and clinical data of propensity score–matched patients did not differ significantly between the NA and control groups.
The main limitations of this study include the hospital-based population and its retrospective nature. Although our hospital is located in a region of 400,000 inhabitants and is the only general hospital visited by a large number of CHB patients, further prospective studies with community-based subjects are warranted. Another limitation was that the propensity score analysis results may be limited by biases related to unmeasured and hidden covariates. Finally, one-to-one matching based on propensity scores resulted in a reduction in the number of patients included.
In conclusion, the survival of patients who received anti-viral NA therapy for CHB was improved compared with that of untreated controls, and NA therapy specifically reduced the risk of HCC mortality. In addition, the causes of death of approximately 40 % of CHB patients who died during follow-up were non-liver-related. Further studies are warranted to confirm these findings in other populations.
Abbreviations
- CHB:
-
Chronic hepatitis B
- HCC:
-
Hepatocellular carcinoma
- HBsAg:
-
Hepatitis B surface antigen
- HBV:
-
Hepatitis B virus
- NA:
-
Nucleos(t)ide analogue
- HBeAg:
-
Hepatitis B e antigen
- ALT:
-
Alanine aminotransferase
- AUC:
-
Area under the curve
- ROC:
-
Receiver operating characteristic
- CI:
-
Confidence interval
- γ-GTP:
-
Gamma-glutamyl transpeptidase
- ALP:
-
Alkaline phosphatase
- AFP:
-
Alphafetoprotein
- HBcrAg:
-
Hepatitis B virus core-related antigen
- BCP:
-
Basal core promoter
- US:
-
Ultrasound
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- IFN:
-
Interferon
- RR:
-
Relative risk
- RFA:
-
Radiofrequency ablation
- PEI:
-
Percutaneous ethanol injection
- TACE:
-
Trancecatheter arterial chemoembolization
- HAIC:
-
Hepatic arterial infusion chemotherapy
References
Lai CL, Ratziu V, Yuen MF, et al. Viral hepatitis B. Lancet. 2003;362:2089–94.
Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107.
Beasley RP, Hwang LY, Lin CC, et al. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129–33.
Szmuness W. Hepatocellular carcinoma and the hepatitis B virus: evidence for a causal association. Prog Med Virol. 1978;24:40–69.
Rustgi VK. Epidemiology of hepatocellular carcinoma. Gastroenterol Clin North Am. 1987;16:545–51.
Kiyosawa K, Umemura T, Ichijo T, et al. Hepatocellular carcinoma: recent trends in Japan. Gastroenterology. 2004;127(Suppl 1):S17–26.
Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–2.
European Association For The Study Of The Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85.
Liaw Yun-Fan, Kao Jia-Horng, Piratvisuth Teerha, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531–61.
Hosaka T, Suzuki F, Kobayashi M, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107.
Kumada T, Toyoda H, Tada T, et al. Effect of nucleos(t)ide analogue therapy on hepatocarcinogenesis in chronic hepatitis B patients: a propensity score analysis. J Hepatol. 2013;58:427–33.
Rosenbaum PR, Rubin DB. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika. 1983;70:41–55.
Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–24.
Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–8.
Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999;150:327–33.
Liaw YF. Natural history of chronic hepatitis B virus infection and long-term outcome under treatment. Liver Int. 2009;29(Suppl 1):100–7.
Chen CJ, Yang HI, Su J, et al. REVEAL-HBV study group risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73.
Yuen MF, Yuan HJ, Wong DK, et al. Prognostic determinants for chronic hepatitis B in asians: therapeutic implications. Gut. 2005;54:1610–4.
Kumada T, Toyoda H, Kiriyama S, et al. Incidence of hepatocellular carcinoma in patients with chronic hepatitis B virus infection who have normal alanine aminotransferase values. J Med Virol. 2010;82:539–45.
Hosmer DW, Lemeshow S. Applied logistic regression. New York: John Wiley & Sons; 2000.
Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36.
Kato H, Orito E, Sugauchi F, et al. Determination of hepatitis B virus genotype G by polymerase chain reaction with hemi-nested primers. J Virol Methods. 2001;98:153–9.
Kimura T, Rokuhara A, Matsumoto A, et al. New enzyme immunoassay for detection of hepatitis B virus core antigen (HBcAg) and relation between levels of HBcAg and HBV DNA. J Clin Microbiol. 2003;41:1901–6.
Wong DK, Tanaka Y, Lai CL, et al. Hepatitis B virus core-related antigens as markers for monitoring chronic hepatitis B infection. J Clin Microbiol. 2007;45:3942–7.
Liu CJ, Chen PJ, Lai MY, et al. Evolution of precore/core promoter mutations in hepatitis B carriers with hepatitis B e antigen seroreversion. J Med Virol. 2004;74:237–45.
Kao JH, Wu NH, Chen PJ, et al. Hepatitis B genotypes and the response to interferon therapy. J Hepatol. 2000;33:998–1002.
The Japan Society of Hepatology. Surveillance algorithm and diagnostic algorithm for hepatocellular carcinoma : Clinical Practice Guidelines for Hepatocellular Carcinoma. Hepatology Res. 2010;40(Supplement s1):6–7.
Shen L, Li JQ, Zeng MD, et al. Correlation between ultrasonographic and pathologic diagnosis of liver fibrosis due to chronic virus hepatitis. World J Gastroenterol. 2006;12:1292–5.
Iacobellis A, Fusilli S, Mangia A, et al. Ultrasonographic and biochemical parameters in the non-invasive evaluation of liver fibrosis in hepatitis C virus chronic hepatitis. Aliment Pharmacol Ther. 2005;22:769–74.
Caturelli E, Castellano L, Fusilli S, et al. Coarse nodular US pattern in hepatic cirrhosis: risk for hepatocellular carcinoma. Radiology. 2003;226:691–7.
World Health Organization, The ICD-10 classification of mental and behavioural disorders : clinical descriptions and diagnostic guidelines.(1992).
Lai CL. Therapeutic advances in chronic hepatitis B. J Gastroenterol Hepatol. 2004;19(Suppl):S5–9.
Leung N. Chronic hepatitis B-treatment with nucleoside analogues. Med J Malaysia. 2005;60(Suppl):22–7.
Takeda A, Jones J, Shepherd J, et al. A systematic review and economic evaluation of adefovir dipivoxil and pegylated interferon-alpha-2a for the treatment of chronic hepatitis B. J Viral Hepat. 2007;14:75–88.
Chen G, Lin W, Shen F, et al. Chronic hepatitis B virus infection and mortality from non-liver causes: results from the Haimen City cohort study. Int J Epidemiol. 2005;34:132–7.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tada, T., Kumada, T., Toyoda, H. et al. Long-term prognosis of patients with hepatitis B infection: causes of death and utility of nucleos(t)ide analogue therapy. J Gastroenterol 50, 795–804 (2015). https://doi.org/10.1007/s00535-014-1011-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-014-1011-6