Abstract
Background
The aim of this multicenter retrospective study was to evaluate the impact of the eradication of hepatitis C virus (HCV) on the clinical outcomes of patients with hepatocellular carcinoma (HCC) treated with molecular-targeted agents (MTAs).
Methods
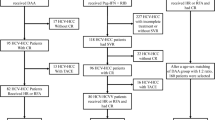
Among 877 patients who received any MTA as first-line systemic therapy for HCC between June 2009 and March 2019, 569 patients with HCV-related HCC were enrolled in this retrospective study. Of these, 109 patients achieved sustained virological response (SVR) before starting MTA. After propensity score matching, the clinical outcomes of 109 patients in the SVR group and 109 patients in the non-SVR group were compared.
Results
The median time to progression in the SVR group (7.8 months) was similar to that in the non-SVR group (5.6 months) (p = 0.212). The median time to treatment failure in the SVR group (5.3 months) was longer than that in the non-SVR group (2.8 months) (p = 0.059), and post-progression survival and overall survival in the SVR group were significantly longer than those in the non-SVR group (12.0 months vs 7.2 months; p = 0.039, and 18.1 months vs 11.3 months; p = 0.019). At the end of first-line MTA therapy, the albumin–bilirubin (ALBI) score in the SVR group ( – 2.25) was significantly lower than that in the non-SVR group ( – 2.10) (p = 0.008).
Conclusions
The eradication of HCV before MTA therapy maintained liver function and led to a prolonged treatment period and improved overall survival of HCV-related HCC patients. We should not overlook the benefits of HCV eradication in HCC patients.





Similar content being viewed by others
Abbreviations
- HCV:
-
Hepatitis C virus
- HCC:
-
Hepatocellular carcinoma
- MTA:
-
Molecular targeted agent
- SVR:
-
Sustained virological response
- TTP:
-
Time to progression
- TTF:
-
Time to treatment failure
- PPS:
-
Post-progression survival
- OS:
-
Overall survival
- AE:
-
Adverse event
- ALBI score:
-
Albumin–bilirubin score
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
References
Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Prim. 2016;2:16018.
Nagaoki Y, Aikata H, Miyaki D, et al. Clinical features and prognosis in patients with hepatocellular carcinoma that developed after hepatitis C virus eradication with interferon therapy. J Gastroenterol. 2011;46:799–808.
Tsuji K, Kurosaki M, Itakura J, et al. Real-world efficacy and safety of ledipasvir and sofosbuvir in patients with hepatitis C virus genotype 1 infection: a nationwide multicenter study by the Japanese Red Cross Liver Study Group. J Gastroenterol. 2018;53:1142–50.
Charlton M, Everson GT, Flamm SL, et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology. 2015;149:649–59.
Kwo PY, Poordad F, Asatryan A, et al. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1–6 without cirrhosis. J Hepatol. 2017;67:263–71.
Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–37.
Shinkawa H, Hasegawa K, Arita J, et al. Impact of sustained virological response to interferon therapy on recurrence of hepatitis C virus-related hepatocellular carcinoma. Ann Surg Oncol. 2017;24:3196–202.
Joko K, Goto T, Watanabe H, et al. Effects of antiviral therapy C following treatment of hepatocellular carcinoma: survey findings of the Japanese Red Cross Liver Study Group. Hepatol Res. 2016;46:251–8.
Miyatake H, Kobayashi Y, Iwasaki Y, et al. Effect of previous interferon treatment on outcome after curative treatment for hepatitis C virus-related hepatocellular carcinoma. Dig Dis Sci. 2012;57:1092–101.
Tanimoto Y, Tashiro H, Aikata H, et al. Impact of pegylated interferon therapy on outcomes of patients with hepatitis C virus-related hepatocellular carcinoma after curative hepatectomy. Ann Surg Oncol. 2012;19:418–25.
Reig M, Marino Z, Perello C, et al. Unexpected high rate of early tumor recurrence in patients with HCV related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719–26.
Waziry R, Hajarizadeh B, Grebely J, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: a systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67:1204–12.
Saraiya N, Yopp AC, Rich NE, et al. Systematic review with meta-analysis: recurrence of hepatocellular carcinoma following direct-acting antiviral therapy. Aliment Pharmacol Ther. 2018;48:127–37.
EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
Bupathi M, Kaseb A, Meric-Bernstam F, et al. Hepatocellular carcinoma: Where there is unmet need. Mol Oncol. 2015;9:1501–9.
Kokudo N, Takemura N, Hasegawa K, et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49:1109–13.
Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–70.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
Terashima T, Yamashita T, Takata N, et al. Post-progression survival and progression- free survival in patients with advanced hepatocellular carcinoma treated by sorafenib. Hepatol Res. 2016;46:650–6.
Hidaka H, Nakazawa T, Kaneko T, et al. Portal hemodynamic effects of sorafenib in patients with advanced hepatocellular carcinoma: a prospective cohort study. J Gastroenterol. 2012;47:1030–5.
Saeki I, Yamasaki T, Yamauchi Y, et al. skeletal muscle volume is an independent predictor of survival after sorafenib treatment failure for hepatocellular carcinoma. Cancers (Basel). 2021;7:2247.
Hiraoka A, Kumada T, Atsukawa M, et al. Important clinical factors in sequential therapy including lenvatinib against unresectable hepatocellular carcinoma. Oncology. 2019;97:277–85.
Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–8.
Hiraoka A, Kumada T, Michitaka K, et al. Usefulness of albumin–bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:1031–6.
Hiraoka A, Kumada T, Kudo M, et al. Albumin–bilirubin (ALBI) grade as part of the evidence-based clinical practice guideline for HCC of the Japan Society of Hepatology: a comparison with the liver damage and Child-Pugh classifications. Liver Cancer. 2017;6:204–15.
Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–28.
Naito S, Tsukamoto T, Murai M, et al. Overall survival and good tolerability of long-term use of sorafenib after cytokine treatment: final results of a phase II trial of sorafenib in Japanese patients with metastatic renal cell carcinoma. BJU Int. 2011;108:1813–9.
Cammà C, Di Bona D, Schepis F, et al. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology. 2004;39:333–42.
Singh S, Facciorusso A, Loomba R, et al. Magnitude and kinetics of decrease in liver stiffness after antiviral therapy in patients with chronic hepatitis C: a systematic review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:27–38.
Sugimoto R, Iwasa M, Hara N, et al. Changes in liver function and body composition by direct-acting antiviral therapy for hepatitis C virus infection. Hepatol Res. 2018;48:337–44.
Takehara T, Sakamoto N, Nishiguchi S, et al. Efficacy and safety of sofosbuvir-velpatasvir with or without ribavirin in HCV-infected Japanese patients with decompensated cirrhosis: an open label phase 3 trial. J Gastroenterol. 2019;54:87–95.
Carrat F, Fontaine H, Dorival C, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019;393:1453–64.
Funding
Authors did not receive any financial support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Yuya Seko, Michihisa Moriguchi, Aya Takahashi, Hidemi Unozawa, Kazufumi Kobayashi, Sadahisa Ogasawara, Rui Sato, Shunji Watanabe, Naoki Morimoto, Satoshi Tsuchiya, and Kenji Iwai. The first draft of the manuscript was written by Yuya Seko and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Sadahisa Ogasawara received honoraria from Bayer, Eisai, Eli Lilly, Chugai Pharma, AstraZeneca, and Merck & Co., Inc., consulting or advisory fees from Bayer, Eisai, Merck & Co., Inc., Chugai Pharma, Eli Lilly, and AstraZeneca, and research grants from Bayer, AstraZeneca, and Eisai. Michihisa Moriguchi received honoraria from Eisai, Bayer, Eli Lilly, and Chugai Pharma, and consulting or advisory fees from Eisai, Bayer, Eli Lilly, and Chugai Pharma. Naoki Morimoto received research grants from Eisai and AbbVie. Takeshi Aramaki received speaker’s bureau fees from Eli Lilly, Chugai Pharma, Termo, Eisai, and Taiho Pharma. Yoshito Itoh received speaker’s bureau fees from MSD, Gilead Sciences, and Abbvie GK, and research grants from MSD, Bristol Myers Squibb, and Scholarship grants from Takeda Pharmaceutical Company Limited, Eisai, Bayer, and Bristol Myers Squibb. Naoya Kato received honoraria from Bayer, Eisai, Sumitomo Dainippon Pharma, and Merck & Co., Inc., consulting or advisory fees from Bayer and Eisai, and research grants from Bayer and Eisai. The other authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Seko, Y., Moriguchi, M., Takahashi, A. et al. Hepatitis C virus eradication prolongs overall survival in hepatocellular carcinoma patients receiving molecular-targeted agents. J Gastroenterol 57, 90–98 (2022). https://doi.org/10.1007/s00535-021-01837-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-021-01837-5