Abstract
Context
Pain is a common experience in people living with cancer. Concerns around opioid prescribing have seen a move toward a multi-modality management approach, which includes interventional pain procedures.
Purpose
In this paper we discuss the interventional pain procedures used to treat cancer pain at two major tertiary centers in Australia.
Methods and results
This expert review provides practical insights on cancer pain management from healthcare providers in different specialties. These insights can be used to guide the management of a wide range of cancer pain types.
Conclusions
Furthermore, this review identifies the need for a systematic and comprehensive approach to the management of cancer pain that is broader than that of a single specialty. With recent advances in pain management procedures, an interdisciplinary approach is essential in order to provide an up to date, patient tailored approach to pain management.
This review will help inform the development of a cancer pain intervention registry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer pain
Pain is a common experience in people living with cancer; up to 80% of patients with advanced cancer experience pain [1]. Studies showed that more than one third of individuals report moderate to severe pain and up to 10% of patients experience chronic severe pain [2]. Cancer pain can be attributed to the malignancy, its complications, or side effects of treatments (e.g., post-surgical pain and chemotherapy-induced peripheral neuropathy) [3, 4]. The underlying pathogenesis may involve not only the nociceptive mechanism caused by ongoing tissue damage, but also neuropathic and sensitization processes caused by atypical somatosensory processing in the peripheral or central nervous systems [4, 5].
In 1985, the World Health Organization proposed the analgesic ladder [6], which supported commencing the treatment of cancer pain with non-opioid medications, before progressing to weak opioids followed lastly by strong opioids. However, both the opioid crisis and the fact that patients can become unresponsive to strong opioids necessitated the re-examination of the use of opioids in the treatment of pain, including cancer pain [7, 8]. The analgesic ladder has subsequently been modified over time, with Vargas-Schaffer suggesting the inclusion of a fourth step which added invasive techniques to the pain management armament (Fig. 1) [8, 9].
With both advances in imaging technology and a better understanding of cancer pain pathogenesis, there has been growing utilization of interventional techniques in the management of cancer pain. The use of advanced imaging modalities has facilitated more and more accurate target localization with less invasive techniques than in the past. In the last few decades, interventions relied initially on landmarks and later on two-dimensional fluoroscopy with X-ray guidance. With growing availability and portability of CT scans, much better precision is achievable with three-dimensional real-time visualization. It also further facilitates treatment of smaller structures such as spinal nerve targets and allows better localization of structures in those with difficult anatomy due to prior surgical procedures or presence of malignancy distorting normal anatomy.
Pain prevention
All patients undergoing cancer treatment should undergo prehabilitation strategies, where possible [10]. Total body prehabilitation can improve post-operative pain. For example, psychological prehabilitation prior to surgery can reduce post-operative pain in people undergoing breast surgery [11], and pre-operative exercise programs in this setting may also reduce post operative pain [12].
Interventional pain procedures
Interventional pain procedures refer to treatments directly applied on a neuraxial region, autonomic nerve plexuses, or peripheral nerves [13]. This may involve the delivery of a drug (often with local anesthetic or neurolytic agents), modulation of nerve transmission through electrical impulses with radiofrequency ablation device or use of spinal cord stimulator, or destructive procedures such as cordotomy [13, 14]. However, apart from celiac plexus blocks, high-quality evidence supporting the use of most of these procedures is lacking, with most evidence derived from retrospective studies or case series conducted in single health institution [15,16,17,18].
Here we explore the range of interventional procedures performed in two major cancer centers in New South Wales, Australia. The interventional pain procedures used to treat cancer pain are outlined in Table 1.
Peripheral nerve blocks
Peripheral nerve blocks work by interrupting nociceptive transmission from a regional nerve innervating the painful structure by use of a local anesthetic injection. Common applications include intercostal nerve blocks for rib pain from bony metastasis, trigeminal nerve blocks for head and neck malignancy, femoral/sciatic nerve blocks for lower limb pain, or serratus anterior blocks for chest wall pain following mastectomy or thoracic surgery. For longer duration of effect lasting from days to weeks, an indwelling catheter for repeated bolus doses or continuous infusion may be considered.
In patients with progressive pain, neurolytic blocks with injection of alcohol or phenol are often the intervention of choice for their more definitively destructive mechanism on affected tissues and nervous structures [19, 20]. The main concerns of peripheral nerve neurolysis include neuritis, painful dysesthesia, or deafferentation pain worsening existing symptoms, and motor weakness or bowel/bladder incontinence if motor/autonomic nerves are involved [19, 20]. The effectiveness and rate of adverse events with peripheral nerve intervention in cancer pain management require further evaluation as most evidence has been derived from case series [19, 20].
Nerve plexus/sympathetic blocks
Interruption of afferent or sympathetic nerve pathways forms the basis for analgesia in relevant body parts. Visceral afferent nociception from cancer involving upper abdominal structures such as the pancreas, liver, stomach, adrenals and kidneys travels through the celiac plexus and then through the splanchnic nerves to the central nervous system.
For lower abdominal and pelvic structures such as the bladder, prostate, sigmoid colon, uterus, ovaries, vaginal fundus, and rectum, the afferent nociceptive signals pass through the superior hypogastric plexus. For perineal structures such as the anus, distal rectum, urethra, scrotum, vulva, and vagina, afferent signals pass through the ganglion impar (ganglion of Walther) located on the anterior surface of the sacrum at the sacrococcygeal junction [21].
Among cancer pain interventions, celiac plexus block and neurolysis have been most heavily studied, and their use has been largely supported by randomized controlled trials and systematic review for analgesic efficacy [22, 23]. Most of the evidence, however, draws on the early studies using a percutaneous fluoroscopy guided approach. Later studies demonstrated non-inferiority from ultrasound-guided modality [24]. Advancing techniques has seen the increasing utilization of CT guided approach to provide three-dimensional visualization of needle placement [25] (Figs. 2 and 3). These advances necessitate the involvement of an interventional radiologist in the interdisciplinary team.
Other sympathetic blocks that can be performed include stellate ganglion blocks for managing pain in the head and neck, breast, and upper limb region and also lumbar sympathetic blocks for sympathetically maintained lower limb pain [26, 27]. Similarly, randomized controlled studies that assess these are lacking in the literature. Splanic nerve block with fluoroscopy/X-ray guidance is shown in Fig. 4.
Intrathecal analgesia
Intrathecal analgesia involves the delivery of medication into cerebrospinal fluid in the vicinity of the intended spinal level/s . Intrathecal drug delivery has been increasingly utilized over the last few decades in patients with cancer that does not respond to conventional medical management or where there are intolerable side-effects systemic analgesic agents. The use of intrathecal opioid analgesia is well supported by reviews on safety, pain reduction, and cost-effectiveness and may be supplemented with other agents off label including local anesthetics or clonidine [28, 29].
The drug/s may be delivered through a single bolus injection, through external infusion pump, or a fully implantable device. The choice of delivery method depends largely on the clinical situation and local nursing expertise and availability. External infusion pumps are usually considered for patients with a limited prognosis measured in weeks, whereas fully implantable devices are more appropriate for patients with a life expectancy greater than 6 months [28, 29].
For cancer patients with poor prognosis and poor functional status, a saddle block, involving administration of neurolytic agents such as alcohol or phenol, can be a helpful procedure for perineal pain. It produces a more prolonged clinical effect at the expense of motor and autonomic functions [28, 29]. Careful patient positioning by an experienced interventionalist is essential, for example sitting with a slight backwards lean when using heavy phenol or in the prone Trendelenburg position when using hypobaric alcohol to avoid spreading to adjacent sensorimotor nerves.
The other potential intervention includes use of a spinal cord stimulator by placement of an electrical lead in the epidural space [30]. However, apart from an associated reduction in opioid use, there is very limited evidence of effectiveness in the management of cancer pain [30].
Cordotomy
Cordotomy is an ablative procedure of the spinal cord (predominately the spinothalamic tract) performed almost exclusively for cancer-related pain. Unilateral percutaneous cordotomy with entry at the level of C1/2 on the contralateral side to the pain is the most common approach, although it can also be performed endoscopically, transdiscally, or as an open procedure [31].
Percutaneous cordotomy requires the patient to be awake and cooperative to allow for sensory stimulation and feedback, whereas the open procedure is done under general anesthesia (Fig. 5). Percutaneous cordotomy is indicated for those with poorly controlled unilateral pain (below the level of the C4 dermatome) and with an estimated prognosis of less than 6 to 12 months [32]. The overall complication rates with CT guidance are low, with severe respiratory compromise and motor weakness being the major concerns.
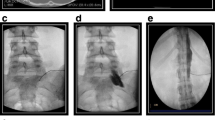
Percutaneous cordotomy procedure for treatment of right hip and leg pain caused by metastatic NSCLC (non-small cell lung cancer) in the pelvic region. A A needle with pulse generator is placed over left neck region for real-time sensory testing to precisely localise the area of pain with CT image guidance. B Radiofrequency device providing thermal lesioning of the spinothalamic fibers. C CT image inside theatre guiding needle placement through C1/2 vertebral space into targeted spinal cord
For those with axial pain below the diaphragm, a bilateral thoracic cordotomy is more appropriate [18]. This requires the expertise of neurosurgeons as an open procedure. Similarly, its use is supported by many case series [33, 34].
Radiofrequency ablation
Radiofrequency ablation is a high temperature thermal therapy that induces coagulation necrosis [35]. It is performed under image guidance and is a useful treatment particularly for patients with painful vertebral metastases [36].
Vertebroplasty
Vertebroplasty involves the injection of artificial bone cement and an opacifier into the inter-trabecular marrow space of fractured vertebrae [37]. It is performed with imaging guidance under local anesthesia [37, 38]. It is useful for painful vertebral compression fractures caused by metastases or multiple myeloma [37, 38] and can be used in patients receiving adjuvant radiation, surgical therapy, or chemotherapy [37]. In Australia, vertebroplasty is performed infrequently due to its invasive nature and lack of clear evidence of its benefit.
Trans-arterial embolization
Trans-arterial embolization involves the injection of embolic agents in the arteries supplying the tumor and is well known for management of spinal bone lesions. There is emerging evidence, primarily based on retrospective studies, demonstrating the potential benefits for treatment of painful bony metastases refractory to conventional radiotherapy [39, 40].
Barriers to cancer pain management
It has long been recognized that cancer pain is undertreated, despite the availability of evidence-based guidelines. An Australian survey of palliative care specialists (n = 92) identified the following barriers to the provision of pain management: insufficient access to nonpharmacologic interventions, patient comorbidities, and a lack of coordination between services [41]. A systematic review of service delivery models for cancer pain recommended the development of policies for referrals to a pain consultation service which can be integrated into a clinical pathway [42]. This would help to address the lack of coordination between services.
A recent qualitative study to identify barriers and facilitators of interventional management of cancer pain interviewed healthcare professionals (n=24) involved in cancer care at two cancer centers in Sydney (unpublished). The study identified six themes, which overlapped with the previous findings. These were (1) clinicians’ knowledge and awareness of interventional pain management, (2) training and resources on interventional pain procedures, (3) unclear referral pathways, (4) clinician perceptions of safety and efficacy, (5) the timing of the intervention, and (6) the need for holistic healthcare. Anecdotally, clinical practices appear to vary significantly due to different expertise and institutional preferences.
A broader review of barriers to optimal cancer pain management identified not only clinician barriers but also society’s attitude toward pain management, system barriers, patient barriers, and healthcare disparities [43]. With regard to healthcare disparities, we know that cancer incidence is higher in New South Wales, Australia outside of the major cities, of which Sydney is one [44]. It is highly likely that access to interventional pain management approaches are limited for patients living with cancer in regional and remote parts of Australia.
Another older Australian study explored patient barriers to optimal pain management. It identified three barriers: (1) poor levels of patient knowledge about pain, (2) low perceived control over pain, and (3) a lack of communication about pain [45]. Without knowing more about how cancer pain is currently being managed it is hard to suggest strategies to overcome these barriers. However, patient education about pain management should not be discounted.
Chronic pain
As cancer survivorship improves, it is important to be aware of the possibility of chronic pain. Cancer survivors often experience adverse physical and psychosocial effects from the diagnosis and its treatment, and should not be overlooked when taking a chronic pain management approach [46, 47]. Cancer survivors with chronic pain also report lack of sleep, fatigue, and mental health issues [47]. In our experience some cancer survival present for repeated interventional pain management procedures, for example, intercostal radiofrequency ablation for persistent post-mastectomy, or post sternotomy pain [48]. Cancer survivors should be assessed and be appropriately referred for treatment of chronic pain, where appropriate. Currently there is very limited evidence to specifically address chronic pain issues in this unique population, and more research into effective strategies is needed.
The need for a cancer pain intervention registry
This review shows the wide range of pain intervention procedures that are currently used in the management of cancer pain. However, to date, there is deficiency of evidence in specific applications, indications, efficacy, or adverse events for varying interventions in treatment of cancer pain. The most recent cancer incidence data for New South Wales, Australia showed 48,165 new cancer diagnoses in 2020 [49]. People are living with cancer for longer than ever before [50], and over one third of cancer patients report moderate to severe cancer pain [2], so the need to understand best practices in cancer pain management is growing.
The establishment of a national cancer pain intervention registry should be considered. This would provide insight into the different interventional pain procedures used in patients with cancer, how these procedures are accessed, their perceived efficacy, and geographical variation. Such a registry would also capture information about the roles and involvement of the interdisciplinary team in the successful management of cancer pain.
Conclusions
This review has provided an overview of interventions used in the management of cancer pain through leading clinicians in the field of pain management and palliative care. It identified need for better evidence guiding future practice, in particular, through establishment of an intervention registry. It also pointed out barriers in achieving optimal pain management, which included lack of co-ordination of suitable interventional pain management in the heterogenous cancer population as one major issue.
Data availability
No datasets were generated or analysed during the current study.
References
Haumann J, Joosten EBA, van den Beuken-van MH (2017) Pain prevalence in cancer patients: status quo or opportunities for improvement? Curr Opin Support Palliat Care 11(2):99–104
Van Den Beuken-Van Everdingen MHJ, Hochstenbach LMJ, Joosten EAJ, Tjan-Heijnen VCG, Janssen DJA (2016) Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manag 51(6):1070–90.e9. https://doi.org/10.1016/j.jpainsymman.2015.12.340
Glare PA, Davies PS, Finlay E, Gulati A, Lemanne D, Moryl N et al (2014) Pain in cancer survivors. J Clin Oncol 32(16):1739
Fallon M, Giusti R, Aielli F, Hoskin P, Rolke R, Sharma M et al (2018) Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol 29:iv166–iiv91
Schmidt BL, Hamamoto DT, Simone DA, Wilcox GL (2010) Mechanism of cancer pain. Mol Interv 10(3):164
Ventafridda V, Saita L, Ripamonti C, De Conno F (1985) WHO guidelines for the use of analgesics in cancer pain. Int J Tissue React 7(1):93–96
Mestdagh F, Steyaert A, Lavand'homme P (2023) Cancer pain management: a narrative review of current concepts, strategies, and techniques. Curr Oncol 30(7):6838–6858. https://doi.org/10.3390/curroncol30070500
Heptonstall N, Scott-Warren J, Berman R, Filippiadis D, Bell J (2023) Role of interventional radiology in pain management in oncology patients. Clin Radiol 78(4):245–253. https://doi.org/10.1016/j.crad.2022.05.022
Vargas-Schaffer G (2010) Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can Fam Physician 56:514–517
Dawson S, Koneti KK (2022) The role of pain management in cancer prehabilitation. In: Chakraborty A, Balakrishnan A (eds) Prehabilitation for cancer surgery. Springer Nature Singapore, Singapore, pp 217–247
Larson MR, Duberstein PR, Talbot NL, Caldwell C, Moynihan JA (2000) A presurgical psychosocial intervention for breast cancer patients. psychological distress and the immune response. J Psychosom Res 48(2):187–194. https://doi.org/10.1016/s0022-3999(99)00110-5
Yang A, Sokolof J, Gulati A (2018) The effect of preoperative exercise on upper extremity recovery following breast cancer surgery: a systematic review. Int J Rehabil Res 41(3):189–196. https://doi.org/10.1097/MRR.0000000000000288
Joshi M, Chambers WA (2010) Pain relief in palliative care: a focus on interventional pain management. Expert Rev Neurother 10(5):747–756
Koyyalagunta D, Simmonds MJ, Novy DM (2019) General pain management concepts. In: Gulati A, Puttanniah V, Bruel BM, Rosenberg WS, Hung JC (eds) Essentials of interventional cancer pain management. Springer, Cham, pp 47–54
Stearns LJ, Narang S, Albright RE, Hammond K, Xia Y, Richter HB et al (2019) Assessment of health care utilization and cost of targeted drug delivery and conventional medical management vs conventional medical management alone for patients with cancer-related pain. JAMA Netw Open 2(4):e191549–e19154e
Hung JC, Azam N, Puttanniah V, Malhotra V, Gulati A (2014) Neurolytic transversus abdominal plane block with alcohol for long-term malignancy related pain control. Pain Physician 17(6):E755–EE60
Vayne-Bossert P, Afsharimani B, Good P, Gray P, Hardy J (2016) Interventional options for the management of refractory cancer pain—what is the evidence? Support Care Cancer 24:1429–1438
Fonoff ET, Lopez WO, de Oliveira YS, Teixeira MJ (2016) Microendoscopy-guided percutaneous cordotomy for intractable pain: case series of 24 patients. J Neurosurg 124(2):389–396. https://doi.org/10.3171/2014.12.Jns141616
Hao D, Fiore M, Di Capua C, Gulati A (2022) Ultrasound-guided peripheral nerve blocks: a practical review for acute cancer-related pain. Curr Pain Headache Rep 26(11):813–820. https://doi.org/10.1007/s11916-022-01089-9
Klepstad P, Kurita GP, Mercadante S, Sjøgren P (2015) Evidence of peripheral nerve blocks for cancer-related pain: a systematic review. Minerva Anestesiol 81(7):789–793
Aman MM, Mahmoud A, Deer T, Sayed D, Hagedorn JM, Brogan SE et al (2021) The American Society of Pain and Neuroscience (ASPN) best practices and guidelines for the interventional management of cancer-associated pain. J Pain Res 14:2139–2164. https://doi.org/10.2147/jpr.S315585
Arcidiacono PG, Calori G, Carrara S, McNicol ED, Testoni PA (2011) Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev 2011(3):Cd007519. https://doi.org/10.1002/14651858.CD007519.pub2
Shwita AH, Amr YM, Okab MI (2015) Comparative study of the effects of the retrocrural celiac plexus block versus splanchnic nerve block, C-arm guided, for upper gastrointestinal tract tumors on pain relief and the quality of life at a six-month follow up. Korean J Pain 28(1):22–31. https://doi.org/10.3344/kjp.2015.28.1.22
Bhatnagar S, Joshi S, Rana SP, Mishra S, Garg R, Ahmed SM (2014) Bedside ultrasound-guided celiac plexus neurolysis in upper abdominal cancer patients: a randomized, prospective study for comparison of percutaneous bilateral paramedian vs. unilateral paramedian needle-insertion technique. Pain Pract 14(2):E63–E68. https://doi.org/10.1111/papr.12107
Vig S, Bhan S, Bhatnagar S (2021) Celiac plexus block—an old technique with new developments. Pain Physician 24(5):379–398
Bhaskar AK, Simpson KH (2022) Interventional management of pain in cancer and palliative care. Medicine (Abingdon 1995, UK ed) 50(12):762–766. https://doi.org/10.1016/j.mpmed.2022.09.002
Silverman JE, Gulati A (2018) An overview of interventional strategies for the management of oncologic pain. Pain Manag 8(5):389–403. https://doi.org/10.2217/pmt-2018-0022
Dupoiron D, Duarte R, Carvajal G, Aubrun F, Eldabe S (2022) Rationale and recent advances in targeted drug delivery for cancer pain: is it time to change the paradigm? Pain Physician 25(3):E414–Ee25
Duarte R et al (2023) Effectiveness and safety of intrathecal drug delivery systems for the management of cancer pain: a systematic review and meta-analysis. Neuromodulation: Technology at the Neural Interface 26(6):1126–1141. https://doi.org/10.1016/j.neurom.2022.03.003
Peng L, Min S, Zejun Z, Wei K, Bennett MI (2015) Spinal cord stimulation for cancer-related pain in adults. Cochrane Database Syst Rev 2015(6):Cd009389. https://doi.org/10.1002/14651858.CD009389.pub3
Raslan AM (2005) Percutaneous computed tomography-guided transdiscal low cervical cordotomy for cancer pain as a method to avoid sleep apnea. Stereotact Funct Neurosurg 83(4):159–164. https://doi.org/10.1159/000088992
Kurita GP, Sjøgren P, Klepstad P, Mercadante S (2019) Interventional techniques to management of cancer-related pain: clinical and critical aspects. Cancers (Basel) 11(4):443. https://doi.org/10.3390/cancers11040443
Poolman M, Makin M, Briggs J, Scofield K, Campkin N, Williams M et al (2020) Percutaneous cervical cordotomy for cancer-related pain: national data. BMJ Support Palliat Care 10(4):429–434. https://doi.org/10.1136/bmjspcare-2019-002057
Szylak R, Bhargava D, Pridgeon M, Srinivasaiah R, Vijayendra V, Osman-Farah J (2023) Open thoracic cordotomy for cancer pain with intraoperative neuromonitoring: a case series and critical review of the literature. World Neurosurg 179:e90–e101. https://doi.org/10.1016/j.wneu.2023.08.016
Patti JW, Neeman Z, Wood BJ (2002) Radiofrequency ablation for cancer-associated pain. J Pain 3(6):471–473. https://doi.org/10.1054/jpai.2002.126785
Rosian K, Hawlik K, Piso R (2018) Efficacy assessment of radiofrequency ablation as palliative pain treatment in patients with painful metastatic spinal lesions: a systematic review. Pain Physician 21:E467–E476
Gudugintta M (2006) Vertebroplasty: a new treatment for vertebral compression fractures. Aust Fam Physician 35(5):304–307
Kam NM, Maingard J, Kok HK, Ranatunga D, Brooks D, Torreggiani WC et al (2017) Combined vertebral augmentation and radiofrequency ablation in the management of spinal metastases: an update. Curr Treat Options in Oncol 18(12):74. https://doi.org/10.1007/s11864-017-0516-7
Facchini G, Parmeggiani A, Peta G, Martella C, Gasbarrini A, Evangelisti G et al (2021) The role of percutaneous transarterial embolization in the management of spinal bone tumors: a literature review. Eur Spine J 30(10):2839–2851. https://doi.org/10.1007/s00586-021-06963-5
Heianna J, Makino W, Toguchi M, Iraha T, Ishikawa K, Kusada T et al (2021) Transarterial chemoembolization for the palliation of painful bone metastases refractory to first-line radiotherapy. J Vasc Interv Radiol 32(3):384–392. https://doi.org/10.1016/j.jvir.2020.10.031
Lovell M, Agar M, Luckett T, Davidson PM, Green A, Clayton J (2013) Australian survey of current practice and guideline use in adult cancer pain assessment and management: perspectives of palliative care physicians. J Palliat Med 16(11):1403–1409. https://doi.org/10.1089/jpm.2013.0245
Brink-Huis A, Tv A, Schoonhoven L (2008) Pain management: a review of organisation models with integrated processes for the management of pain in adult cancer patients. J Clin Nurs 17(15):1986–2000. https://doi.org/10.1111/j.1365-2702.2007.02228.x
Scarborough BM, Smith CB (2018) Optimal pain management for patients with cancer in the modern era. CA Cancer J Clin 68(3):182–196. https://doi.org/10.3322/caac.21453
Cancer Institute NSW: Cancer incidence and mortality—remoteness/socioeconomic position. https://www.cancer.nsw.gov.au/research-and-data/cancer-data-and-statistics/data-available-now/cancer-statistics-nsw/cancer-incidence-and-mortality (2023). Accessed 27 July 2023.
Yates PM, Edwards HE, Nash RE, Walsh AM, Fentiman BJ, Skerman HM et al (2002) Barriers to effective cancer pain management: a survey of hospitalized cancer patients in Australia. J Pain Symptom Manag 23(5):393–405. https://doi.org/10.1016/s0885-3924(02)00387-1
Glare P, Aubrey K, Gulati A, Lee YC, Moryl N, Overton S (2022) Pharmacologic management of persistent pain in cancer survivors. Drugs 82(3):275–291. https://doi.org/10.1007/s40265-022-01675-6
Gallaway MS, Townsend JS, Shelby D, Puckett MC (2020) Pain among cancer survivors. Prev Chronic Dis 17:E54. https://doi.org/10.5888/pcd17.190367
Yasin J, Thimmappa N, Kaifi JT, Avella DM, Davis R, Tewari SO et al (2020) CT-guided cryoablation for post-thoracotomy pain syndrome: a retrospective analysis. Diagn Interv Radiol 26(1):53–57. https://doi.org/10.5152/dir.2019.19179
Cancer Institute NSW: cancer type summaries—incidence. https://www.cancer.nsw.gov.au/research-and-data/cancer-data-and-statistics/data-available-now/cancer-statistics-nsw/cancer-type-summaries (2023). Accessed 27 July 2023.
Cancer Institute NSW: cancer type summaries—survival. https://www.cancer.nsw.gov.au/research-and-data/cancer-data-and-statistics/data-available-now/cancer-statistics-nsw/cancer-type-summaries (2023). Accessed 27 July 2023.
Acknowledgements
The authors thank Pippa Burns, PhD, CMPP of WriteSource Medical Pty Ltd, Sydney, Australia, for providing medical writing support. Medical writing support was funded by Chris O’Brien Lifehouse SurFebruary Cancer Research Fund in accordance with Good Publication Practice (GPP2022) guidelines (https://www.acpjournals.org/doi/10.7326/M22-1460).
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This project is supported by Chris O’Brien Lifehouse SurFebruary Cancer Research Fund for employment of a part time research assistant over 2-year periods.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by Yi-Ching Lee and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Relevance of manuscript to inform research, policies, and/or programs
This study describes the types of interventional approaches used in cancer pain management. Further work is required to understand the types of patients that access this type of pain management and barriers to care.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, YC., Brake, T., Zhao, E. et al. The use of interventional procedures for cancer pain. A brief review. Support Care Cancer 32, 285 (2024). https://doi.org/10.1007/s00520-024-08467-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-024-08467-6