Abstract
Purpose
This systematic review updates the MASCC/ESMO recommendations for high-emetic-risk chemotherapy (HEC) published in 2016–2017. HEC still includes cisplatin, carmustine, dacarbazine, mechlorethamine, streptozocin, and cyclophosphamide in doses of > 1500 mg/m2 and the combination of cyclophosphamide and an anthracycline (AC) in women with breast cancer.
Methods
A systematic review report following the PRISMA guidelines of the literature from January 1, 2015, until February 1, 2023, was performed. PubMed (Ovid), Scopus (Google), and the Cochrane Database of Systematic Reviews were searched. The literature search was limited to randomized controlled trials, systematic reviews, and meta-analyses.
Results
Forty-six new references were determined to be relevant. The main topics identified were (1) steroid-sparing regimens, (2) olanzapine-containing regimens, and (3) other issues such as comparisons of antiemetics of the same drug class, intravenous NK1 receptor antagonists, and potentially new antiemetics. Five updated recommendations are presented.
Conclusion
There is no need to prescribe steroids (dexamethasone) beyond day 1 after AC HEC, whereas a 4-day regimen is recommended in non-AC HEC. Olanzapine is now recommended as a fixed part of a four-drug prophylactic antiemetic regimen in both non-AC and AC HEC. No major differences between 5-HT3 receptor antagonists or between NK1 receptor antagonists were identified. No new antiemetic agents qualified for inclusion in the updated recommendations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The risk of nausea and vomiting following antineoplastic therapy depends on the emetic risk potential of the antineoplastic therapy, patient demographics such as sex (women are at a higher risk than men) and age (younger patients have a higher risk than older), and the antiemetic prophylaxis prescribed.
The emetic risk of antineoplastic agents administered intravenously (i.v.) is defined as the risk of vomiting within the first 24 h after the start of antineoplastic therapy in patients who did not receive antiemetic prophylaxis. High emetic risk is defined as a risk of more than 90% of vomiting. High-emetic-risk antineoplastic agents administered intravenously include cisplatin, carmustine, dacarbazine, mechlorethamine, streptozocin, and cyclophosphamide in doses of > 1500 mg/m2 and the combination of cyclophosphamide and an anthracycline (AC) in women with breast cancer. All these are chemotherapeutic agents and are referred to as high-emetic-risk chemotherapy (HEC).
Very few data on the emetic risk potential of orally administered antineoplastic agents exist, and the emetic risk potential refers to the risk during the entire treatment period rather than the first 24 h.
This manuscript is a systematic review and update of the MASCC/ESMO recommendations for high-emetic-risk antineoplastic agents published in 2016–2017 [1, 2].
Methods
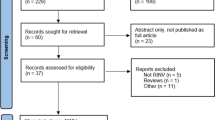
A literature search was conducted from January 1, 2015, through February 1, 2023. PubMed (Ovid), Scopus (Google), and the Cochrane Database of Systematic Reviews were searched. The reporting of literature search followed the PRISMA guidelines [3]. The seven HEC agents were used as keywords and paired with each of the available antiemetics within the five antiemetic drug groups (neurokinin (NK)1 receptor antagonists, serotonin (5-HT)3 receptor antagonists, corticosteroids, dopamine (D)2,3 receptor antagonists, and cannabinoids). For example, the search terms for cisplatin were as follows: cisplatin AND aprepitant OR netupitant OR rolapitant OR fosaprepitant OR fosnetupitant OR neurokinin antagonist; cisplatin AND ondansetron OR granisetron OR palonosetron OR ramosetron OR serotonin antagonist; cisplatin AND dexamethasone or methylprednisolone or prednisolone or steroid; cisplatin AND metoclopramide OR domperidone OR metopimazine OR prochlorperazine OR olanzapine OR amisulpride OR dopamine antagonist; cisplatin AND cannabis OR tetrahydrocannabinol OR nabilone OR dronabinol OR cannabidiol OR cannabinoid. The search was limited to randomized controlled trials, systematic reviews, and meta-analyses. The number of results identified from literature search and determined to be relevant is summarized as a PRISMA flow diagram in Fig. 1. For the distribution of selected references in each of the antiemetic drug groups, see Table 1.
Results
A total of 80 references were identified as relevant for further full-text review after reading abstracts of all 1058 references. The 80 references were distributed as follows: NK1 receptor antagonist (n = 25), 5-HT3 receptor antagonists (n = 35), D2,3 receptor antagonists (n = 14), corticosteroids (n = 6), and cannabinoids (n = 0). After removal of duplicates, 46 references qualified for consideration by the guideline committee, in the context of updating this guideline [2, 4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. The references were divided into three categories according to the quality of the study and the potential to change the guideline.
-
Category 1 [4, 9, 12, 13, 20, 22, 24, 25, 30, 34, 38, 39, 41, 44, 48]:
-
References have the potential to change the guidelines and are described in detail in the manuscript.
-
Category 2 : [5-8, 10, 11, 14, 15, 17-19, 21, 23, 26-28, 31-33, 35-37, 40, 42, 43, 45, 47]:
-
References are supportive for category 1 references and are described briefly in the manuscript.
-
Category 3 [3, 16, 29, 46]:
-
References about new agents or minor studies in new settings may be hypothesis generating. These references are mentioned in the manuscript.
The main topics identified were (1) steroid-sparing regimens, (2) olanzapine-containing regimens, and (3) other issues such as comparisons of antiemetics of the same drug class, intravenous NK1 receptor antagonists, and potentially new antiemetics.
Steroid-sparing regimens
The literature search identified six references qualifying for inclusion in the current update. These included two original studies [8, 22], a combined analysis of these two studies [7], a sub-analysis [6] of one of the studies [8], and two meta-analysis [5, 31] of which one [31] included a systematic review. A meta-analysis from 2019 including eight studies concluded that a single day of dexamethasone (DEX) is as good as a 3-day regimen in patients receiving moderately emetogenic chemotherapy (MEC) or AC chemotherapy [5]. Another systematic review and meta-analysis from 2019 including five studies and using a non-inferiority margin of −8% confirmed non-inferiority of a 1-day DEX regimen compared to a 3-day DEX regimen in MEC and AC patients [31]. This was further investigated in a randomized, double-blinded, placebo-controlled, non-inferiority trial including 396 patients [22]. Patients in this trial received cisplatin-based (> 50 mg/m2) or AC chemotherapy. Patients were randomized to receive either DEX day 1 (12 mg i.v.) plus placebo days 2–3 or DEX 12 mg i.v. day 1 followed by DEX 8 mg days 2–3. All patients also received palonosetron 0.75 mg i.v. plus aprepitant 125 mg p.o. day 1 followed by 80 mg p.o. days 2–3 or fosaprepitant 150 mg i.v. day 1. Patients were stratified for age and chemotherapy (cisplatin versus AC). The primary end point was complete response (CR) in the overall period (defined as no emetic episodes and no use of rescue medication days 1–5 after chemotherapy), and the non-inferiority margin was 15%. CR was 46.9% (3 days of DEX) versus 44% (1 day of DEX), p = 0.007 (95% CI, −12.6 to 6.8%). A subgroup analysis of patients receiving AC confirmed non-inferiority of the 1-day DEX regimen, whereas non-inferiority was not confirmed in patients receiving cisplatin-based chemotherapy. In a multicenter, randomized, open-designed, non-inferiority, three-arm study, Celio et al. investigated chemotherapy-naïve patients who received their first course of cisplatin-based (> 70 mg/m2) chemotherapy [8]. All patients received oral NEPA (netupitant 300 mg plus palonosetron 0.5 mg) and DEX 12 mg i.v. before chemotherapy and were randomized to no DEX days 2–4 (DEX1), oral DEX 4 mg × 1 days 2–3 (DEX3), or oral DEX 4 mg × 2 on days 2–4 (DEX4). The primary endpoint was CR (defined as above) in the overall phase. The study was powered (80%) not to overlook differences larger than 15%. Non-inferiority was confirmed for the DEX1 arm compared to the DEX4 arm (95% CI, −12.3 to 15%). The authors reported that a limitation was the open design and that only 33% of the patients were women. Furthermore, the overall CR in the control arm was lower (75%) than estimated in the patient sample size calculation (90%). It is important to note that none of the above studies included olanzapine as an antiemetic. It may be possible that the addition of olanzapine to a three-drug DEX-sparing regimen would be non-inferior to a conventional 4-day DEX regimen in patients treated with cisplatin. In fact the SPARED study [29] suggests that this may be possible; although results were presented as a late breaking abstract at the annual ESMO congress in 2021 [49], full publication is not available at the time of the update (September 2023).
Olanzapine-containing regimens
Nine references evaluating olanzapine qualified for inclusion in the update. These consisted of two systematic reviews [2, 18] and seven randomized, controlled trials [11, 13, 21, 30, 38, 41, 43] of which all but one [43] used a double-blind design.
Olanzapine as an add-on to a three-drug regimen
Two large phase 3 studies compared the addition of olanzapine to the standard antiemetic regimen of a 5-HT3 receptor antagonist plus DEX plus an NK1 receptor antagonist. Navari et al. completed a randomized, double-blind trial comparing a 5-HT3 receptor antagonist plus DEX plus aprepitant/fosaprepitant plus placebo with the same 3-drug regimen plus oral olanzapine 10 mg once daily on days 1–4 after chemotherapy. The study included 380 chemotherapy-naïve patients receiving either cisplatin-based (> 70 mg/m2) or AC chemotherapy [30]. Patients were stratified for sex, chemotherapy (cisplatin-based versus AC), and the specific 5-HT3 receptor antagonist (palonosetron, granisetron, and ondansetron). The primary end point was no nausea (defined as 0 mm on a visual analogue scale during the overall assessment period from 0 to 120 h after chemotherapy). CR (defined as no emetic episodes and no need of rescue medication from 0 to 120 h after chemotherapy), no acute nausea (0–24 h), and no delayed nausea (24–120 h) were all secondary endpoints. No nausea was significantly more frequent in the olanzapine group than in the placebo group, with no nausea rates of 74% versus 45% (0–24 h, p = 0.002), 42% versus 25% (24–120 h, p = 0.002), and 37% versus 22% (0–120 h, p = 0.002). Also the number of patients with CR was significantly higher in the olanzapine group (86% versus 65%, 67% versus 52%, and 64% versus 41% in the acute, delayed, and overall phases, respectively. Sedation was more frequent in the olanzapine group, but both antiemetic regimens were well tolerated. Hashimoto et al. completed a similar double-blind study in chemotherapy-naïve patients receiving cisplatin-based (> 50 mg/m2) chemotherapy, but used olanzapine 5 mg daily for 4 days (instead of 10 mg as in the Navari study), and all patients received palonosetron (0.75 mg × 1 i.v) as the preferred 5-HT3 receptor antagonist in combination with DEX and aprepitant/fosaprepitant [12, 13]. The study included 705 evaluable patients, and stratification was done for sex, dose of cisplatin, and age. The primary end point was CR in the delayed phase (24–120 h after cisplatin). Olanzapine significantly improved the number of patients with CR in the delayed phase ([79%; 95% CI 75–83] versus [66%; 95% CI 61–71], p < 0.0001), but also in the acute and overall phases (secondary end points). Furthermore, the number of patients obtaining complete control (defined as CR and no more than mild nausea) and total control (defined as CR and no nausea) was also significantly higher in the olanzapine group. Sedation was not significantly more frequent in the olanzapine group, and the authors concluded that this was due to the lower dose of olanzapine and the administration after dinner (instead of the usual dosing in the morning). A third, randomized, double-blind study (n = 208) compared olanzapine 5 mg p.o daily for 4 days with placebo in chemotherapy-naïve patients with breast cancer receiving four cycles of neoadjuvant or adjuvant AC (90%) or cyclophosphamide (non-anthracycline)-based (10%) chemotherapy. All patients, in addition to olanzapine/placebo, received aprepitant, ondansetron, and DEX. The primary end point was self-reported nausea and secondary end points were control of acute and delayed nausea and vomiting [11]. Olanzapine significantly reduced the number of patients reporting nausea during all four cycles (27.7% versus 41.3%, p < 0.001), whereas the number of vomiting episodes was not statistically significantly reduced. Mild sedation was more frequent in the olanzapine group (54.1% versus 40.8%, p < 0.001).
Finally, a randomized, open, study (n = 120) in chemotherapy-naïve Chinese breast cancer patients receiving neoadjuvant or adjuvant AC chemotherapy compared aprepitant, ondansetron, and DEX with or without the addition of olanzapine 10 mg p.o. once daily for 5 days [43]. The authors concluded that addition of olanzapine increased the number of patients with CR (no vomiting and no use of rescue medication), the rates of no nausea (nausea on a visual analogue scale (VAS) < 5 mm), and no significant nausea (nausea VAS < 25 mm).
Dose and schedule of olanzapine
The most frequent adverse effect of olanzapine is sedation which could be severe in older patients [50]. The vast majority of studies (n ≈ 30) have investigated olanzapine in a dose and schedule of 10 mg once daily for 4 days usually administered during daytime [10, 30, 43, 51, 52]. A number of studies (n ≈ 15) have investigated olanzapine in a dose of 5 mg once daily [13, 48, 53, 54], and in some of these (n ≈ 10), olanzapine was administered at bedtime to avoid or diminish sedation [13, 48]. A few studies (n ≈ 10) have compared 5 mg and 10 mg of olanzapine [21, 38, 41], but none of these studies used guideline-recommended methodology or included a sufficient number of patients in order to conclude the benefits and harms between 5 mg and 10 mg [55]. A review from 2022 concluded that the evidence for administration at bedtime remains weak [56], and still no comparisons with daytime administration have been done.
Other issues
Comparison of different 5-HT3 receptor antagonists
Two studies compared the 5-HT3 receptor antagonist ramosetron with palonosetron [23] and ondansetron [26], respectively. In a single-blind non-inferiority study, 279 patients were treated with cisplatin-based (72%) or AC-based (28%) chemotherapy, and all received aprepitant (days 1–3) and DEX (days 1–4) for antiemetic protection and were randomized to ramosetron 0.3 mg i.v. or palonosetron 0.25 mg i.v. on day 1. Ramosetron was non-inferior to palonosetron, with respect to the primary end point of complete response (no emesis and no rescue antiemetics in the first 5 days after chemotherapy) and all secondary end points. No differences in adverse events were observed [23]. In another single-blind study [26] with a similar design, 299 patients treated with cisplatin-based or AC-based chemotherapy all received aprepitant and DEX and were randomized to ramosetron 0.3 mg i.v. or ondansetron 16 mg i.v. on day 1. Ramosetron was non-inferior to ondansetron, but the interpretation of the results was confounded by a significant difference in the number of women allocated to ramosetron (20.8%) and ondansetron (41.9%), because it is well known that the female sex increases the risk of CINV.
Two studies compared outcomes between granisetron and palonosetron [27, 40]. In a randomized, double-blind trial, 842 patients treated with cisplatin-based (> 50 mg/m2) chemotherapy all received aprepitant (days 1–3) and DEX (days 1–4) and were randomized to palonosetron 0.75 mg i.v. (day 1) or granisetron 1 mg i.v. (on day 1). The study had 90% power to detect differences larger than 10%. The primary end point was CR (no emesis and no rescue antiemetics) in the first 120 h after chemotherapy. CR was not statistically significant different between the palonosetron (65.7%) and granisetron (59.1%) arms (95% CI 1.35 (0.99–1.82), p = 0.0539). A number of secondary end points favored palonosetron in the delayed phase (24–120 h after cisplatin), but differences were all less than 10% [40]. A randomized, double-blind study compared the effect of palonosetron 0.75 mg i.v. on day 1 against granisetron 1 mg i.v. on day 1, with both arms combined with fosaprepitant 150 mg i.v. on day 1 and DEX days 1–3 in women with breast cancer treated with AC-based chemotherapy [27]. The study included 326 patients, and the primary end point was CR (no emesis and no rescue antiemetics) in the delayed phase (24–120 h after chemotherapy). No significant differences in CR (24–120 h) were seen (CR granisetron 60.4% versus 62.3% palonosetron, p = 0.8) or in acute (0–24 h) or overall CR (0–120 h). An open, randomized study with a high dropout rate (18.3%) concluded that granisetron (transdermal administration) was non-inferior to ondansetron i.v., and both arms combined with aprepitant and DEX in patients receiving highly emetogenic chemotherapy [39].
A systematic review and meta-analysis published in 2021 [20] included 12 studies and concluded that palonosetron was superior to granisetron, but in a sub-analysis of the only three studies [27, 40, 57] including an NK1 receptor antagonist, this advantage disappeared with the exception of a minor advantage of palonosetron CR in the delayed phase (95% CI 1.30 (1.02–1.64)). However, it should be noted that olanzapine was not included in any of the above studies or in the systematic review.
New studies of i.v. NK1 receptor antagonists and comparison of different NK1 receptor antagonists
There are differences between the intravenous formulations of the NK1 receptor antagonists.
An injectable emulsion of rolapitant was approved by FDA in 2017, but due to serious hypersensitivity reactions [58], the rolapitant emulsion approval was withdrawn in January 2021 [59]. Fosaprepitant was already proven non-inferior to aprepitant and described in the 2016 guidelines [2]. Non-inferiority was recently confirmed in two large studies in Chinese patients receiving HEC, primarily cisplatin-based chemotherapy [42, 60]. Fosaprepitant induces injection site reactions (ISRs) in a small number of patients, in particular those receiving AC-based chemotherapy. Another intravenous formulation of aprepitant (HTX-019, an injectable emulsion of aprepitant free of polysorbate 80)) has a lower incidence of ISRs [59, 61, 62]. In a large phase 2 study (i = 584), fosnetupitant (two different doses) was compared with placebo both combined with palonosetron and DEX in patients receiving cisplatin-based (> 70 mg/m2) chemotherapy [37]. The high dose of fosnetupitant (235 mg) significantly improved the antiemetic effect of palonosetron and DEX as compared to placebo, and no significant differences in adverse events were observed. This confirmed results from a previous study by Hesketh et al. already reviewed in the 2016 guidelines [2]. Schwartzberg and colleagues compared intravenous NEPA (fosnetupitant and i.v. palonosetron) with oral NEPA both combined with DEX in two randomized, double-blind studies in patients receiving cisplatin-based (n = 404) and AC-based (n = 402) chemotherapy, respectively [35, 36]. The primary end point was safety and tolerability, and both studies included a multiple cycle extension (n = 4). It was concluded that there was no difference between i.v. and oral NEPA as concerns antiemetic efficacy or safety. It is noteworthy, that no significant differences were observed in ISRs.
Three studies compared a (fos)netupitant-based regimen against a (fos)aprepitant-based antiemetic regimen [14, 28, 45]. In a large randomized, double-blind, non-inferiority, phase 3 study (n = 828), oral NEPA and DEX were compared to aprepitant, granisetron, and DEX in patients receiving cisplatin-based (> 50 mg/m2) chemotherapy [45]. The primary end point was CR (defined as no emesis and no rescue antiemetics) during the first 120 h after start of cisplatin. Non-inferiority was demonstrated for acute CR (0–24 h), delayed CR (24–120 h), overall CR (0–120 h), and for no emesis; no nausea (< 5 mm on a 0-100 mm VAS) and no significant nausea (< 25 mm) both in the acute, delayed, and overall phases. A secondary (preplanned) analysis of the Chinese subpopulation (80.6%) confirmed these results [9]. Another randomized, double-blind, non-inferiority, phase 3 study (n = 785) compared fosnetupitant with fosaprepitant both combined with palonosetron and DEX in patients receiving cisplatin-based (> 70 mg/m2) chemotherapy [14]. Non-inferiority was proven for all efficacy end points. There were no differences in adverse effects with the exception of ISR, which was more frequently observed with fosaprepitant. Finally, a small randomized, double-blind, phase 3 study (n = 102) compared fosnetupitant with fosaprepitant both combined with palonosetron and DEX in patients treated with AC/EC chemotherapy [28]. The primary end point was the incidence of treatment-related adverse events (TRAEs), whereas efficacy end points were secondary. No significant differences in TRAEs were seen with the exception of TRAEs relevant for ISRs observed in 0% of the fosnetupitant patients, compared to 10% of fosaprepitant patients. It should be noted that none of the above studies compared fosnetupitant with HTX-019 aprepitant emulsion that has a lower risk of ISRs than fosaprepitant [59, 61, 62].
Potential new antiemetics
A few studies have investigated other drugs for the protection of nausea and vomiting in HEC [4, 16, 46]. In a randomized, double-blind, placebo-controlled, dose-ranging, phase 2 study (n = 318), the dopamine D3 receptor antagonist, amisulpride, improved the antiemetic effect of ondansetron in chemotherapy-naïve patients treated with cisplatin-based (> 70 mg/m2) chemotherapy [16]. A single oral dose of 10 mg days 2–4 was significantly superior to placebo as concerns the primary end point, delayed CR (no emesis and no rescue antiemetics 24–120 h after start of chemotherapy) obtained in 46% versus 20% of patients (p = 0.002) and the secondary end point, delayed no nausea rate (< 5 mm on a 100 mm VAS) obtained in 37% versus 19% (p = 0.016). No significant differences in adverse effects (including sedation) was seen. An open-label study (n = 100, closed prematurely due to slow recruitment) investigated the antiemetic effect of the atypical tetracyclic antidepressant, mirtazapine, with affinity for multiple receptors (serotonin, histamine, adrenergic). The study indicated that mirtazapine can improve the effect of aprepitant, palonosetron, and DEX on delayed emesis in women treated with cisplatin-based chemotherapy or EC and who experienced delayed emesis in the preceding chemotherapy cycle [4].
Thalidomide was investigated in a large randomized, double-blind trial (n = 638) in chemotherapy-naïve patients scheduled to receive their first course of cisplatin-based (> 50 mg/m2) or AC/EC chemotherapy [46]. Patients received palonosetron on day 1 and DEX on days 1–4 and were randomized to oral thalidomide 100 mg twice daily on days 1–5 or placebo. The primary end point was CR (25–120 h after start of chemotherapy). Thalidomide significantly improved the rates of CR in the delayed and overall phases (76.9% versus 61.7%, p < 0.001 and 66.1% versus 53.3%, p = 0.001, respectively). Dizziness, constipation, sedation, and dry mouth were adverse events more frequently observed with thalidomide, whereas insomnia was more frequent in the placebo-treated patients.
Discussion
This systematic review is the result of a literature search, reported in accordance with the PRISMA guidelines for systematic reviews, and the review and discussions of the references relevant for the guideline update. One face-to-face meeting and five virtual meetings provided the background for the literature review and update of the guideline recommendations. The recommendations are summarized in Table 2.
There is level I evidence to limit dosing of dexamethasone to day 1 after AC chemotherapy. For patients receiving cisplatin-based (and other non-AC HEC), results are inconclusive and the 2016 recommendation of a 3–4 day DEX regimen stands. None of the studies defined in the literature search included olanzapine, except for the SPARED study [29, 49]; however, at the time of this review writing (September 2023), it is published as an abstract only and therefore not considered in this update. It is possible, that the addition of olanzapine makes it possible to limit the administration of dexamethasone to day 1 also in patients receiving cisplatin-based chemotherapy [63].
The addition of olanzapine to a three-drug regimen of a 5-HT3 receptor antagonist an NK1 receptor antagonist and DEX was optional in the 2016 MASCC/ESMO guidelines. Recently, large, well-conducted studies [13, 30] delivered clear evidence that olanzapine improves outcomes of the above three-drug regimen and olanzapine is now recommended as a fixed part of a four-drug regimen. This is in line with the ASCO recommendations [17, 18]. Sedation is an adverse event and could be a problem in older patients. Therefore, lower doses of olanzapine and administration at bedtime have been investigated. Unfortunately comparative studies (of olanzapine 10 mg and 5 mg) are few and not sufficiently powered to conclude if the 5-mg dose is as effective as the 10-mg dose [64] (Table 2). No new significant differences between the 5-HT3 receptor antagonists have been disclosed in this review. It is possible that palonosetron exhibits a small advantage in the protection of delayed nausea and vomiting if an NK1 receptor antagonist is not available or affordable [20].
Across the different NK1 receptor antagonists, no new difference were disclosed. This means that there are minor differences in the pharmacology (e.g., half-life and risk of drug-drug interactions), but this has not resulted in major differences in the effect or tolerability. The i.v. formulations of fosnetupitant [14, 28] and the HTX-019 emulsion of aprepitant [59, 61, 62] both seem to have a very low risk of ISRs.
No new antiemetics qualified for inclusion in the guideline update. Two agents (amisulpride and mirtazapine) were investigated and seemed to possess antiemetic efficacy in HEC patients, but none of the studies included guideline-recommended antiemetic regimens [4, 16]. A third study concluded that thalidomide improves the effect palonosetron and DEX in patients treated with HEC, but again an NK1 receptor antagonist (or olanzapine) was not included and concerns about adverse events have been raised [65].
Finally, although not part of this review, it is concluded that in spite of the major contribution from olanzapine in reducing nausea, this adverse event remains the major CINV problem in HEC patients.
References
RRoila F, Molassiotis A, Herrstedt J, Aapro M, Gralla RJ, Bruera E, Clark-Snow RA, Dupuis LL, Einhorn LH, Feyer P, Hesketh PJ, Jordan K, Olver I, Rapoport BL, Roscoe J, Ruhlmann CH, Walsh D, Warr D, van der Wetering M on behalf of the participants of the MASCC/ ESMO Consensus Conference Copenhagen (2015) 2016 MASCC/ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 27(Supplement 5):v119–v133. https://doi.org/10.1093/annonc/mdw270
Herrstedt J, Roila F, Warr D, Celio L, Navari RM, Hesketh PJ, Chan A, Aapro MS (2017) 2016 updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following high emetic risk chemotherapy. Support Care Cancer 25:277–288. https://doi.org/10.1007/s00520-016-3313-0
Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, Koffel JB, PRISMA-S Group (2021) PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev 10:39. https://doi.org/10.1186/s13643-020-01542-z
Cao J, Ouyang Q, Wang S, Ragaz J, Wang X, Teng Y, Wang B, Wang Z, Zhang J, Wang L, Wu J, Shao Z, Hu X (2020) Mirtazapine, a dopamine receptor inhibitor, as secondary prophylactic for delayed nausea and vomiting following highly emetogenic chemotherapy; an open label, randomized, multicenter phase III trial. Invest New Drugs 38:507–514. https://doi.org/10.1007/s10637-020-00903-8
Celio L, Bonizzoni E, Zattarin E, Codega P, de Braud F, Aapro M (2019) Impact of dexamethasone-sparing regimens on delayed nausea caused by moderately and highly emetogenic chemotherapy: a meta-analysis of randomized evidence. BMC Cancer 19:1268. https://doi.org/10.1186/s12885-019-6454-y
Celio L, Cortinovis D, Cogoni AA, Cavanna L, Martelli O, Carnio S, Collovà E, Bertolini F, Petrelli F, Cassano A, Chiari R, Zanelli F, Pisconti S, Vittimberga I, Letizia A, Misino A, Gernone A, Bonizzoni E, Pilotto S et al (2022) Evaluating the impact of chemotherapy-induced nausea and vomiting on daily functioning in patients receiving dexamethasone-sparing regimens with NEPA (netupitant/palonosetron) in the cisplatin setting: results from a randomized phase 3 study. BMC Cancer 22:915. https://doi.org/10.1186/s12885-022-10018-3
Celio L, Bonizzoni E, Montani E, Aapro M (2022) Efficacy of the dexamethasone-sparing triplet regimen for preventing cisplatin-induced emesis: a combined analysis. Future Oncol 18:3389–3397. https://doi.org/10.2217/fon-2022-0330
Celio L, Cortinovis D, Cogoni AA, Cavanna L, Martelli O, Carnio S, Collovà E, Bertolini F, Petrelli F, Cassano A, Chiari R, Zanelli F, Pisconti S, Vittimberga I, Letizia A, Misino A, Gernone A, Bonizzoni E, Pilotto S et al (2021) Dexamethasone-sparing regimens with oral netupitant and palonosetron for the prevention of emesis caused by high-dose cisplatin: a randomized noninferiority study. Oncologist 26:e1854–e1861. https://doi.org/10.1002/onco.13851
Chang J, Chen G, Wang D, Wang G, Lu S, Feng J, Li W, Li P, Lanzarotti C, Chessari S, Zhang L (2020) Efficacy of NEPA, a fixed antiemetic combination of netupitant and palonosetron, vs a 3-day aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting (CINV) in Chinese patients receiving highly emetogenic chemotherapy in a randomized phase 3 study. Cancer Med 9:5134–5142. https://doi.org/10.1002/cam4.3123
Clemmons AB, Orr J, Andrick B, Gandhi A, Sportes C, DeRemer D (2018) Randomized, placebo-controlled, phase III trial of fosaprepitant, ondansetron, dexamethasone (FOND) versus FOND plus olanzapine (FOND-O) for the prevention of chemotherapy-induced nausea and vomiting in patients with hematologic malignancies receiving highly emetogenic chemotherapy and hematopoietic cell transplantation regimens: The FOND-O trial. Biol Blood Marrow Transplant 24:2065–2071. https://doi.org/10.1016/j.bbmt.2018.06.005
Clemons M, Dranitsaris G, Sienkiewicz M, Sehdev S, Ng T, Robinson A, Mates M, Hsu T, McGee S, Freedman O, Kumar V, Fergusson D, Hutton B, Vandermeer L, Hilton J (2020) A randomized trial of individualized versus standard of care antiemetic therapy for breast cancer patients at high risk for chemotherapy-induced nausea and vomiting. Breast 54:278–285. https://doi.org/10.1016/j.breast.2020.11.002
Hashimoto H, Abe M, Yanai T, Yamaguchi T, Zenda S, Uchitomi Y, Fukuda H, Mori M, Iwasa S, Yamamoto N, Ohe Y (2018) Study protocol for J-SUPPORT 1604 (J-FORCE): a randomized, double-blind, placebo-controlled phase III study evaluating olanzapine (5 mg) plus standard triple antiemetic therapy for prevention of chemotherapy induced nausea and vomiting in patients receiving cisplatin-based highly emetogenic chemotherapy. Jpn J Clin Oncol 48:950–952. https://doi.org/10.1093/jjco/hyy114
Hashimoto H, Abe M, Tokuyama O, Mizutani H, Uchitomi Y, Yamaguchi T, Hoshina Y, Sakata Y, Takahashi TY, Nakashima K, Nakao M, Takei D, Zenda S, Mizukami K, Iwasa S, Sakurai M, Yamamoto N, Ohe Y (2020) Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicenter, randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 21:242–249. https://doi.org/10.1016/S1470-2045(19)30678-3
Hata A, Okamoto I, Inui N, Okada M, Morise M, Akiyoshi K, Takeda M, Watanabe Y, Sugawara S, Shinagawa N, Kubota K, Saeki T, Tamura T (2022) Randomized, double-blind phase III study of fosnetupitant versus fosaprepitant for prevention of highly emetogenic chemotherapy-induced nausea and vomiting: CONSOLE. J Clin Oncol 40:180–188. https://doi.org/10.1200/JCO.21.01315
He Mc XR, Liu M, Zhang Y, Yi F, Wei Y, Liu Q, Zhang W (2021) Use of dexamethasone and a 5-HT3 receptor antagonist with or without aprepitant to prevent chemotherapy-induced nausea and vomiting among patients with lung cancer who are treated with platinum-based chemotherapy: a systematic review and meta-analysis of randomized controlled trials. Ann. Palliat Med 10:4308–4319. https://doi.org/10.21037/apm-20-2290
Herrstedt J, Summers Y, Jordan K, Pawel J von, Jakobsen AH, Ewertz M, Chan S, Naik JD, Karthaus M, Dubey S, Davis R, Fox GM. Amisulpride prevents nausea and vomiting associated with highly emetogenic chemotherapy: a randomized, double-blind, placebo-controlled, dose-ranging trial. Support Care Cancer 2019 27:2699-2705. https://doi.org/10.1007/s00520-018-4564-8
Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, Danso MA, Dennis K, Dupuis LL, Dusetzina SB, Eng C, Feyer PC, Jordan K, Noonan K, Sparacio D, Somerfield MR, Lyman GH (2017) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. Clin Oncol 35:3240–3261. https://doi.org/10.1200/JCO.2017.74.4789
Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, Danso MA, Dennis K, Dupuis LL, Dusetzina SB, Eng C, Feyer PC, Jordan K, Noonan K, Sparacio D, Somerfield MR, Lyman GH (2020) Antiemetic: ASCO guideline update. J Clin Oncol 38:2782–2797. https://doi.org/10.1200/JCO.20.01296
Hesketh PJ, Palmas M, Nicolas P (2018) Preventing chemotherapy-induced nausea and vomiting in patients with lung cancer: efficacy of NEPA (netupitant-palonosetron), the first combination antiemetic. Support Care Cancer 26:1151–1159. https://doi.org/10.1007/s00520-017-3936-9
Hsu Y-C, Chen C-Y, Tam K-W (2021) Effectiveness of palonosetron versus granisetron in preventing chemotherapy-induced nausea and vomiting: a systematic review and meta-analysis. Eur J Clin Pharmacol 77:1597–1609. https://doi.org/10.1007/s00228-021-03157-2
Ithimakin S, Theeratrakul P, Laocharoenkiat A, Nimmannit A, Akewanlop C, Soparattanapaisarn N, Techawattanawanna S, Korphaisarn K, Danchaivijitr P (2020) Randomized, double-blind, placebo-controlled study of aprepitant versus two dosages of olanzapine with ondansetron plus dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving high-emetogenic chemotherapy. Support Care Cancer 28:5335–5342. https://doi.org/10.1007/s00520-020-05380-6
Ito Y, Tsuda T, Minatogawa H, Kano S, Sakamaki K, Ando M, Tsugawa K, Kojima Y, Fujuruya N, Matsuzaki K, Fukuda M, Sugae S, Ohta I, Arioka H, Tokuda Y, Narui K, Tsuboya A, Suda T, Morita S et al (2018) Placebo-controlled, double-blinded, phase III study comparing dexamethasone on day 1 with dexamethasone on days 1 to 3 with combined neurokinin-1 receptor antagonist and palonosetron in high-emetogenic chemotherapy. J Clin Oncol 36:1000–1006. https://doi.org/10.1200/JCO.2017.74.4375
Kang JH, Kwon JH, LeeY-G PKU, An HJ, Sohn J, Seol YM, Lee H, Yun H-J, Ahn JS, Yang JH, Song H, Koo D-H, Kim JY, Kim GM, Kim HJ (2020) Ramosetron versus palonosetron in combination with aprepitant and dexamethasone for the control of highly-emetogenic chemotherapy-induced nausea and vomiting. Cancer Res Treat 52:907–916. https://doi.org/10.4143/crt.2019.713
Karthaus M, Voisin D, Rizzi G, Ciuleanu T (2020) Phase 3 study of palonosetron iv infusion vs iv bolus for chemotherapy-induced nausea and vomiting prophylaxis after highly emetogenic chemotherapy. J Pain Sympt Manag 60:568–576. https://doi.org/10.1016/j.jpainsymman.2020.03.034
Karthaus M, Tibor C, Lorusso V, Singh-Arora R, Filippov A, Rizzi G, Borroni ME, Rossi G, Grunberg SM (2015) Efficacy and safety of oral palonosetron compared with iv palonosetron administered with dexamethasone for the prevention of chemotherapy-induced nausea and vomiting (CINV) in patients with solid tumors receiving cisplatin-based highly emetogenic chemotherapy (HEC). Support Care Cancer 23:2917–2923. https://doi.org/10.1007/s00520-015-2657-1
Kim JH, Shin SW, Song E-K, Lee N-R, Kim JS, Ahn JS, Yun H-J, Cho Y-H, Park KU, Kim S-Y, Jang JS, Kim S-W, Lee HW, Lee SR, Kim YS, Lee SN, Ko YH, Kim HJ, Kang J-H (2015) Ramosetron versus ondansetron in combination with aprepitant and dexamethasone for the prevention of highly emetogenic chemotherapy-induced nausea and vomiting: a multicenter, randomized, phase III trial, KCSG PC10-21. Oncologist 20:1440–1447. https://doi.org/10.1634/theoncologist.2015-0128
Matsumoto K, Takahashi M, Sato K, Osaki A, Takano T, Naito Y, Matsuura K, Aogi K, Fujiwara K, Tamura K, Baba M, Tokunaga S, Hirano G, Imoto S, Miyazaki C, Yanagihara K, Imamura CK, Chiba Y, Saeki T (2020) A Double-blind, randomized, multicenter phase 3 study of palonosetron vs granisetron combined with dexamethasone and fosaprepitant to prevent chemotherapy-induced nausea and vomiting in patients with breast cancer receiving anthracycline and cyclophosphamide. Cancer Med 9:3319–3327. https://doi.org/10.1002/cam4.2979
Matsuura K, Tsurutani J, Inoue K, Tanabe Y, Taira T, Kubota K, Tamura T, Saeki T (2022) A phase 3 safety study of fosnetupitant as an antiemetic in patients receiving anthracycline and cyclophosphamide. Cancer 128:1692–1698. https://doi.org/10.1002/cncr.34088
Minatogawa H, Izawa N, Kawaguchi T, Miyaji T, Shimomura K, Kazunori H, Lihara H, Ohno Y, Inada Y, Arioka H, Morita H, Hida N, Sugawara M, Katada C, Nawata S, Ishida H, Tsuboya A, Tsuda T, Yamaguchi T, Nakajima TE (2020) Study protocol for SPARED trial: randomized, non-inferiority phase III trial comparing dexamethasone on day 1 with dexamethasone on days 1-4, combined with neurokinin-1 receptor antagonist, palonosetron and olanzapine (5 mg) in patients receiving cisplatin-based chemotherapy. BMJ Open 17(10):e041737. https://doi.org/10.1136/bmjopen-2020-041737
Navari RM, Qin R, Ruddy KJ, Liu H, Powell SF, Bajaj M, Dietrich L, Biggs D, Lafky JM, Loprinzi CL (2016) Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. New Engl J Med 375:134–142. https://doi.org/10.1056/NEJMoa1515725
Okada Y, Oba K, Furukawa N, Kosaka Y, Okita K, Yuki S, Komatsu Y, Celio L, Aapro M (2019) One-day versus three-day dexamethasone in combination with palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a systematic review and individual patient data-based meta-analysis. Oncologist 24:1593–1600. https://doi.org/10.1634/theoncologist.2019-0133
Patel P, Leeder JS, Piquette-Miller M, Dupuis LL (2017) Aprepitant and fosaprepitant drug interactions: a systematic review. Br J Clin Pharmacol 83:2148–2162. https://doi.org/10.1111/bcp.13322
Piechotta V, Adams A, Haque M, Scheckel B, Kreuzberger N, Monsef I, Jordan K, Kuhr K, Skoetz N (2021) Antiemetics for adults for prevention of nausea and vomiting by moderately or highly emetogenic chemotherapy: a network meta-analysis (review). Cochrane Database Syst Rev 11:CD012775. https://doi.org/10.1002/14651858.CD012775.pub2
Schnadig ID, Agajanian R, Dakhil C, Gabrail NY, Smith RE Jr, Taylor C, Wilks ST, Schwartzberg LS, Cooper W, Mosier MC, Payne JY, Klepper MJ, Vacirca JL (2016) APF530 (granisetron injection extended release) in a three-drug regimen for delayed CINV in highly emetogenic chemotherapy. Future Oncol 12:1469–1481. https://doi.org/10.2217/fon-2016-0070
Schwartzberg L, Roland E, Andric Z, Kowalski D, Radic J, Voisin D, Rizzi G, Navari R, Gralla RJ, Karthaus M (2018) Phase III safety study of intravenous NEPA: a novel fixed antiemetic combination of fosnetupitant and palonosetron in patients receiving highly emetogenic chemotherapy. Ann Oncol 29:1535–1540. https://doi.org/10.1093/annonc/mdy169
Schwartzberg L, Navari R, Clark-Snow R, Arkania E, Radyukova I, Patel K, Voisin D, Rizzi G, Wickham R, Gralla RJ, Aapro M, Roland E (2020) Phase IIIb safety and efficacy of intravenous NEPA for prevention of chemotherapy-induced nausea and vomiting (CINV) in patients with breast cancer receiving initial and repeat cycles of anthracycline and cyclophosphamide (AC) chemotherapy. Oncologist 25:e589–e597. https://doi.org/10.1634/theoncologist.2019-0527
Sugawara S, Inui N, Kanehara M, Morise M, Yoshimori K, Kumagai T, Fukui T, Minato K, Iwashima A, Takeda Y, Kubota K, Saeki T, Tamura T (2019) Multicenter, placebo-controlled, double-blind, randomized study of fosnetupitant in combination with palonosetron for the prevention of chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Cancer 125:4076–4083. https://doi.org/10.1002/cncr.32429
Sukauichai S, Ketkaew C, Othaganont N, Pichaya P, Promsuwan P (2022) Efficacy of olanzapine 5 mg versus 10 mg for the prophylaxis of chemotherapy-induced nausea and vomiting in patients receiving high emetic risk chemotherapy without neurokinin-1 receptor antagonist. Asian Pac J Cancer Prev 23:2137–2143. https://doi.org/10.31557/APJCP.2022.23.6.2137
Sun DS, Ko YH, Jin JY, Woo IS, Park SY, Eom YA, Kang JH, Kim HK (2022) Efficacy of granisetron transdermal system for the control of nausea and vomiting induced by highly emetogenic chemotherapy: a multicenter, randomized, controlled trial. Korean J Intern Med. https://doi.org/10.3904/kjim.2020.359
Suzuki K, Yamanaka T, Hashimoto H, Shimada Y, Arata K, Matsui R, Goto K, Takiguchi T, Ohyanagi F, Kogure Y, Nogami N, Nakao M, Takeda K, Azuma K, Nagase S, Hayashi T, Fujiwara K, Shimada T, Seki N, Yamamoto N (2016) Randomized, double-blind, phase III trial of palonosetron versus granisetron in the triplet regimen for preventing chemotherapy-induced nausea and vomiting after highly emetogenic chemotherapy: TRIPLE study. Ann Oncol 27:1601–1606. https://doi.org/10.1093/annonc/mdw220
Yanai T, Iwasa S, Hashimoto H, Ohyanagi F, Takiguchi T, Takeda K, Nakao M, Sakai H, Nakayama T, Minato K, Arai T, Suzuki K, Shimada Y, Nagashima K, Terakado H, Yamamoto N (2018) A double-blind randomized phase II dose-finding study of olanzapine 10 mg or 5 mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol 23:382–388. https://doi.org/10.1007/s10147-017-1200-4
Yang LQ, Sun XC, Qin SK, Cheng Y, Shi JH, Chen ZD, Wang QM, Zhang HL, Hu B, Liu B, Zhang QY, Shu YQ, Dong J, Han BH, Wang KM, Dang CX, Li JL, Wang HB, Li BL et al (2017) Efficacy and safety of fosaprepitant in the prevention of nausea and vomiting following highly emetogenic chemotherapy in Chinese people: a randomized, double-blind, phase III study. Eur J Cancer Care 26(6):e12668. https://doi.org/10.1111/ecc.12668
Yeo W, Lau TKH, Li L, Lai KT, Pang E, Cheung M, Chan VTC, Wong A, Soo WMT, Yeong VTY, Tse T, Lam DCM, Yeung EWM, Ng KPK, Tang NLS, Tong M, Suen JJS, Mo FKF (2020) A randomized study of olanzapine versus standard antiemetic regimens for the prevention of chemotherapy-induced nausea and vomiting in Chinese breast cancer patients. Breast 50:30–38. https://doi.org/10.1016/j.breast.2020.01.005
Yokoe T, Hayashida T, Nagayama A, Nakashoji A, Maeda H, Seki T, Takahashi M, Takano T, Abe T, Kitagawa Y (2019) Effectiveness of antiemetic regimens for highly emetogenic chemotherapy‐induced nausea and vomiting: a systematic review and network meta‐analysis. Oncologist 24:e347–e357. https://doi.org/10.1634/theoncologist.2018-0140
Zhang L, Lu S, Feng J, Dechaphunkul A, Chang J, Wang D, Chessari S, Lanzarotti C, Jordan K, Aapro M (2018) A randomized phase III study evaluating the efficacy of single-dose NEPA, a fixed antiemetic combination of neupitant and palonosetron, versus an aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy (HEC). Ann Oncol 29:452–458. https://doi.org/10.1093/annonc/mdx698
Zhang L, Qu X, Teng Y, Shi J, Yu P, Sun T, Jingyan W, Zhu Z, Zhang X, Zhao M, Liu J, Jin B, Luo Y, Teng Z, Dong Y, Wen F, An Y, Yuan C, Chen T et al (2017) Efficacy of thalidomide in preventing delayed nausea and vomiting induced by highly emetogenic chemotherapy: a randomized, multicenter, double-blind, placebo-controlled phase III trial (CLOG1302 study). J Clin Oncol 35:3558–3565. https://doi.org/10.1200/JCO.2017.72.2538
Zhang Z, Zhang Y, Chen G, Hong S, Yang Y, Fang W, Luo F, Chen X, Ma Y, Zhao Y, Zhan J, Xue C, Hou X, Zhou T, Ma S, Gao F, Huang Y, Chen L, Zhou N et al (2018) Olanzapine-based triple regimens versus neurokinin-1 receptor antagonist-based triple regimens in preventing chemotherapy-induced nausea and vomiting associated with highly emetogenic chemotherapy: a network meta-analysis. Oncologist 23:603–616. https://doi.org/10.1634/theoncologist.2017-0378
Zhao Y, Yang Y, Gao F, Hu C, Zhong D, Lu M, Yuan Z, Zhao J, Miao J, Li Y, Zhu J, Wang C, Han J, Zhao Y, Huang Y, Zhang L (2022) A multicenter, randomized, double-blind, placebo-controlled phase 3 trial of olanzapine plus triple antiemetic regimen for the prevention of multiple-day highly emetogenic chemotherapy-induced nausea and vomiting (OFFER study). eClinicalMedicine:101771. https://doi.org/10.2016/j.eclinm.2022.101771
Shimomura K, Minatogawa H, Mashiko T, Arioka H, Lihara H, Sugawara M, Hida N, Akiyama K, Nawata S, Tsuboya A, Mishima K, Izawa N, Miyaji T, Honda K, Inada Y, Ohno Y, Katada C, Morita H, Yamaguchi T, Nakajima TE (2021) Placebo-controlled, double-blinded, phase 3 study comparing dexamethasone on day 1 with dexamethasone on days 1 to 4, with combined neurokinin-1 receptor antagonist, palonosetron, and olanzapine in patients receiving cisplatin-containing highly emetogenic chemotherapy. Ann Oncol 32(supplement 5) s1339,LBA63
Herrstedt J, Lindberg S, Petersen PC (2022) Prevention of chemotherapy-induced nausea and vomiting in the older patient: optimizing outcomes. Drugs Aging 39:1–21. https://doi.org/10.1007/s40266-021-00909-8
Navari RM, Gray SE, Kerr AC (2011) Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 9:188–195. https://doi.org/10.1016/j.suponc.2011.05.002
Mukhopadhyay S, Kwatra G, Alice K P, Badyal D (2017) Role of olanzapine in chemotherapy-induced nausea and vomiting on platinum-based chemotherapy patients: a randomized, controlled study. Support Care Cancer 25:145–154. https://doi.org/10.1007/s00520-016-3386-9
Gao J, Zhao J, Jiang C, Chen F, Zhao L, Jiang Y, Li H, Wang W, Wu Y, Jin Y, Da L, Liu G, Zhang Y, Li H, Zhang Z, Jin G, Li Q (2022) Olanzapine (5 mg) plus standard triple antiemetic therapy for the prevention of multiple-day chemotherapy-induced nausea and vomiting: a prospective randomized controlled study. Support Care Cancer 30:6225–6232. https://doi.org/10.1007/s00520-022-07067-6
Liu G, Jin Y, Jiang Y, Zhao J, Jiang C, Zhang Z, Zhao L, Li H, Chen F, Wang J, Fan H, Li Z, Jia Y, Jin G, Li Q (2022) A comparison of the efficacy 5 mg olanzapine and aprepitant in the prevention of multiple-day cisplatin chemotherapy-induced nausea and vomiting. Int J Clin Practice. https://doi.org/10.1155/2022/5954379
Sutherland A, Naessens K, Plugge E, Ware L, Head K, Burton MJ, Wee B (2018) Olanzapine for the prevention and treatment of cancer-related nausea and vomiting in adults (review). Cochrane Database Syst Rev 9:CD012555. https://doi.org/10.1002/14651858/CD012555.pub2
Zhang X-L, Ying J-E (2022) Olanzapine for the prevention and treatment of chemotherapy-induced nausea and vomiting: a review to identify the best way to administer the drug. Current Oncol 29:8235–8243. https://doi.org/10.3390/curroncol29110650
Kimura H, Yamamoto N, Shirai T, Nishida H, Hayashi K, Tanzawa Y, Takeuchi A, Igarashi K, Inatani H, Shimozaki S, Kato T, Aoki Y, Higuchi T, Tsuchiya H (2015) Efficacy of triplet regimen antiemetic therapy for chemotherapy-induced nausea and vomiting (CINV) in bone and soft tissue sarcoma patients receiving highly emetogenic chemotherapy, and an efficacy comparison of singleshot palonosetron and consecutive-day granisetron for CINV in a randomized, single-blinded crossover study. Cancer Med 4:333–341. https://doi.org/10.1002/cam4.373
Cass AS, Odinet JS, Valgus JM, Crona DJ (2019) Infusion reactions following administration of intravenous rolapitant in an academic center. J Oncol Pharm Pract 25:1776–1783. https://doi.org/10.1177/1078155218808084
Tyler T, Schultz A, Venturini A, Giuliano C, Bernareggi A, Spezia R, Voisin D, Stella V (2022) Challenges in the development of intravenous neurokinin-1 receptor antagonists. Results of a safety and pharmacokinetics dose-finding, phase 1 study of intravenous fosnetupitant. Clin Pharmacol Drug Dev 11:1405–1418. https://doi.org/10.1002/cpdd.1183
Zhao Y, Zhao B, Chen G, Chen Y, Liao Z, Zhang H, Feng W, Li Y, Weng H, Li W, Zhou Y, Ren B, Lu Y, Chen J, Liu Z, Su Z, Wang W, Zhang L (2023) Validation of different personalized risk models of chemotherapy-induced nausea and vomiting: results of a randomized, double-blind, phase III trial of fosaprepitant for cancer patients treated with high-dose cisplatin. Cancer Commun 43:246–256. https://doi.org/10.1002/cac2.12397
Ottoboni T, Lauw M, Keller MR, Cravets M, Manhard K, Clendeninn N, Quart B (2018) Safety of HTX-019 (intravenous aprepitant) and fosaprepitant in healthy subjects. Future Oncol 14:2849–2859. https://doi.org/10.2217/fon-2018-0311
Dranatsaris G, Moezi M, Dobson K, Phelan R, Blau S (2022) A real-world study to evaluate the safety and efficacy of three injectable neurokinin-1 receptor antagonist formulations for the prevention of chemotherapy-induced nausea and vomiting in cancer patients. Support Care Cancer 30:6649–6658. https://doi.org/10.1007/s00520-022-07080-7
Chow R, Herrstedt J, Aapro M, Chiu L, Lam H, Prsic E, Lock M, DeAngelis C, Navari RM (2021) Olanzapine for the prophylaxis and rescue of chemotherapy-induced nausea and vomiting: a systematic review, meta-analysis, cumulative metaanalysis and fragility assessment of the literature. Support Care Cancer 29:3439–3459. https://doi.org/10.1007/s00520-020-05935-7
Chow R, Navari RM, Terry B, DeAngeles C, Prsic EH (2022) Olanzapine 5 mg vs 10 mg for the prophylaxis of chemotherapy-induced nausea and vomiting: a network meta-analysis. Support Care Cancer 30:1015–1018. https://doi.org/10.1007/s00520-021-06606-x
Chong MF, Chan A (2018) Thalidomide for delayed chemotherapy-induced nausea and vomiting: where is its place in therapy? J Clin Oncol 36:827–828. https://doi.org/10.1200/JCO.2017.76.4316
Funding
Open access funding provided by Copenhagen University. The Multinational Association of Supportive Care in Cancer and the European Society for Medical Oncology funded participation in a consensus meeting in Toronto June 2022.
No other funding was received.
Author information
Authors and Affiliations
Contributions
All authors contributed to the literature search.
All authors reviewed the references disclosed from the literature search
In particular the literature on steroids was reviewed by LC and MA, the literature on 5-HT3-receptor antagonists by LZ and JH, the literature on NK1-receptor antagonists by PJH, RC and JH, the literature on dopamine-receptor antagonist by RMN and JH, the literature on cannabinoids by MA and JH and the literature on pharmacological issues by MS and AC.
JH drafted the manuscript. No medical writer was included in the manuscript writing.
All authors commented on the draft manuscript.
All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not relevant
Consent to participate
Not relevant
Competing Interests
Jørn Herrstedt (JH) declares that he has received honoraria from PharmaThen S.A.
Luigi Celio (LC) has received consulting fees Italfarmaco; speaker’s fee Berlin-Chemie AG.
Paul J Hesketh (PJH) declares that he has no financial interests.
Li Zhang (LZ) declares that he has no financial interests.
Rudolph M Navari (RMN) declares that he has no financial interests.
Alexandre Chan (AC) declares that he has no financial interests.
Mitsue Saito (MS) declares that she has no financial interests.
Ronald Chow (RC) declares that he has no financial interests.
Matti Aapro (MA) declares the following interests relevant to this manuscript: has received honoraria from Berlin-Chemie, Fosun, Helsinn Healthcare SA, Juniper Biologics, Knight Therapeutics, Mundipharma International Limited, Vifor Pharma.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Celio L is an independent medical oncologist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Herrstedt, J., Celio, L., Hesketh, P. et al. 2023 updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following high-emetic-risk antineoplastic agents. Support Care Cancer 32, 47 (2024). https://doi.org/10.1007/s00520-023-08221-4
Published:
DOI: https://doi.org/10.1007/s00520-023-08221-4