Abstract
Introduction
High-intensity interval training (HIIT) is an appropriate training modality to improve endurance and therefore contributes to physical performance. This review investigates the effect of HIIT on functional performance in cancer patients. We reviewed the relative peak oxygen uptake (relV̇O2PEAK) and meta-analytical compared HIIT with moderate intensity continuous training (MICT). Furthermore, we took various training parameters under consideration.
Methods
A systematic literature search was conducted in Scopus, PubMed, and Cochrane Library databases. For the review, we included randomized controlled trials containing HIIT with cancer patients. From this, we filtered interventions with additional MICT for the meta-analysis. Outcomes of interest were various functional performance assessments and V̇O2MAX.
Results
The research yielded 584 records which fit the inclusion criteria, of which 31 studies with n=1555 patients (57.4±8.6 years) could be included in the overall review and 8 studies in the meta-analysis (n=268, 59.11±5.11 years) regarding relV̇O2PEAK. Different functional outcomes were found, of which walking distance (+8.63±6.91% meters in 6-min walk test) and mobility (+2.7cm in sit and reach test) improved significantly due to HIIT. In terms of relV̇O2PEAK, the performance of cancer patients was improved by HIIT (10.68±6.48%) and MICT (7.4±4.29%). HIIT can be favored to increase relV̇O2PEAK (SMD 0.37; 95% CI 0.09–0.65; I2=0%; p=0.009). Effect sizes for relV̇O2PEAK improvements correlate moderately with total training volume (Spearman’s ρ=0.49; p=0.03), whereas percentage increases do not (Spearman’s ρ=0.24; p=0.14).
Conclusion
Functional and physical outcomes were positively altered by different HIIT protocols and forms of implementation, whereas a tendency toward more effectiveness of HIIT vs. MICT was found for relV̇O2PEAK. Future studies should include functional parameters more often, to finally allow a comparison between both training protocols in this regard.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adhering to common physical activity guidelines is considered an essential factor in prevention, treatment, and aftercare of various cancers [1], and has been shown to improve cancer-specific survival after treatments and all-cause mortality [2,3,4]. Exercise as a planned, structured, and repetitive subset of physical activity [5] has been shown to contribute to both the prevention and management for several chronic diseases [6], including cancer [7]. In addition to improved physical fitness and maintained activities of daily living, supervised physical training can make an impact on psychological well-being and consequently improve quality of life [7, 8]. Concomitant to medical treatment, exercise may be beneficial to reduce symptom experience (e.g., cancer-related fatigue) and other therapy-related symptoms (e.g., from radiation and pharmaceuticals), and the risk of recurrence can be reduced [7, 9,10,11,12,13].
Cardiovascular diseases (CVD) share a number of risk factors with cancer [14]. A study showed that CVD may be the primary cause of death in breast cancer survivors [15], an interesting finding that requires further evidence for other types of cancer. Studies show that cardiovascular training in cancer patients may be beneficial for multiple dimensions, such as physical function (e.g. V̇O2), cancer-related fatigue, and functional capacity [16,17,18,19]. This gives cardiovascular training (e.g., endurance exercise) a particular relevance for cancer survivors. Consistent with this, higher cardiorespiratory fitness has been associated with reduced cancer mortality [20]. However, while individualized endurance training is recommended as a part of an optimally designed exercise program in cancer patients [21], there is still a lack of consensus as to which type of endurance exercise is most effective. Endurance exercise can be performed continuously with low to moderate intensity (MICT) or intermittently [22]. High-intensity-interval training (HIIT) in particular, consisting of short, high-intensity training sessions (e.g., >80% maximal oxygen consumption [V̇O2MAX]) interspersed with low-intensity recovery phases [23, 24] has gained interest not only in elite sports but also in the therapy of various diseases [25,26,27]. Specific improvements were for example reduced dosage of medication and improved endurance performance in type 2 diabetes patients [25]. HIIT was found to be significantly more effective than MICT to improve cardiac functions in myocardia infarct patients [26].
Milanović et al. [28] found a potentially large positive effect on V̇O2MAX of +5.5±1.2 ml kg−1 min−1 after HIIT compared to healthy controls who did not exercise in young to middle-aged healthy individuals. Moreover, HIIT may have additional benefits as it induces alterations in peripheral muscle tissue (e.g. increased fiber cross sectional area and capillary-to-fiber ratio) that lead to a reduction in adverse effects of training, such as dyspnea and leg discomfort [29]. In a comprehensive meta-analysis, Batacan et al. [30] report a significant improvement in V̇O2MAX through HIIT in normal weight and overweight/obese populations, respectively. Furthermore, HIIT is a highly effective approach to improving cardiorespiratory fitness and quality of life in adults with chronic disease, especially in comparison with other forms of endurance training such as MICT [29, 31, 32].
As a result of early diagnosis and advanced treatment, cancer becomes a chronic disease for many people, with persistent side effects of therapy (e.g., loss of muscle mass and strength, loss of mobility and upper extremity disability, lymphedema, fatigue, and cardiac toxicity) [33]. Functional performance can be impaired by muscle loss, limited upper and lower extremity strength, reduced walking distance, and various physical symptoms [34,35,36,37] from which an essential goal in the cancer aftercare is derived.
Studies indicate that HIIT is more beneficial than MICT for improving functional performance and sustaining those effects after detraining [38, 39]. HIIT can therefore be an efficient training regimen to promote functional performance [38]. Superior effects of HIIT (vs. MICT) were also found for functional mobility in a healthy elderly population [39]. The application of HIIT is acknowledged to be feasible and safe for cancer patients and can be an alternative to conventional endurance training to increase physical capacity [40,41,42]. Due to the stated efficacy in terms of time, HIIT seems suitable for the supportive treatment of chronic diseases [43].
HIIT is a suitable form of training for a broad cancer patient population [42]. HIIT can be performed by various types of cancer in UICC stages I–IV in prehabilitation (e.g., [44]), therapy (e.g., [45]), and aftercare (e.g., [46]). Nevertheless, a combination of HIIT and chemoradiation therapy can lead to an exacerbation of side effects and the subsequent reduction in quality of life [47]. No substantial dropouts were reported even in a population with advanced cancer (stage IV) [48]. High adherence was documented regarding perceived training sessions and targeted intensities [46, 49].
While the positive effects of HIIT on physical fitness in cancer patients have been recognized [42], specific consideration of functional tests is lacking. Those outcomes could be essential to assess effects relevant to the everyday life of cancer survivors. In addition, HIIT protocols seem to be increasingly common in intervention studies from 2019 till now (total database records per year). Therefore, we performed a systematic literature review to analyze the functional performance following HIIT. We analyzed the effects of HIIT on maximal oxygen uptake (V̇O2MAX) and performed the meta-analytic approach comparing HIIT and MICT. In addition, we provide an overview of the specific features of the training programs used in the included studies. Based on the results of the review and meta-analysis, we aim to derive a possible preference regarding HIIT or MICT as a preferred training method in cancer patients.
Methods
Systematic literature search
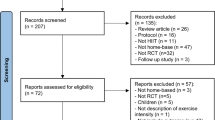
The research was performed in line with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) recommendations [50]. The search terms “cancer” AND “high intensity interval” were used for the systematic literature search. In March 2023, the PubMed, Scopus, and Cochrane Library databases were searched independently by two investigators using the specified search terms. In case of disagreement, a third reviewer was consulted. Information regarding the selection process is shown in the flow chart (Fig. 1).
PRISMA flow chart to illustrate the selection of literature [70]
Eligibility criteria
The eligibility criteria were based on the PICOS framework (population, intervention, comparison, outcome, study design). Studies with adult cancer patients of all types of cancer, stage, and sexes were included. The intervention had to consist solely of HIIT (any interval intensities, durations, and frequencies) over a period of at least 3 weeks, with a control group receiving only medical treatment (e.g., no exercise training, usual care) or another group performing any form of MICT. Considered outcomes were various practical functional assessments (e.g., 6-min walk test (6MWT), timed up and go test (TUG), sit to stand test (STS), sit and reach test (SRT), grip strength (GS), Margaria-Kalamen stair test (MKST), and chair stand test (CST)) and measurements for cardiorespiratory fitness (CRF) V̇O2PEAK or V̇O2MAX. We did not incorporate questionnaires, for instance on physical activity or self-assessments. Only randomized controlled trials (RCTs) with a pre-post design were included.
Data extraction
The following data were extracted: (a) general study information—authors, publication year, study design; (b) subject information: sample size, anthropometrics, cancer-related information (e.g., usual care specifics, type of cancer , surgeries, time since diagnosis), UICC (Union for International Cancer Control) stages; (c) HIIT and MICT intervention data according to FITT criteria (F=Frequency; I=Intensity; T=Time; T=Type) [51]—duration, frequency, intensity, training equipment; (d) outcome parameter: functional assessments (e.g., 6MWT, sit to stand test, grip strength), measurements for CRF (relVO2PEAK, V̇O2MAX). Outcomes were extracted from pre- and post-data of the studies. This was followed by converting the outcome data into the respective percentage change of the parameter. In the case of unspecific data areas (e.g., 70–85%), the respective mean value was used for further calculations. We calculated a total training volume by multiplying training weeks by training frequency per week and the duration of one session in minutes.
If available, data was extracted in terms of mean, standard deviation (SD), and sample size for meta-analysis. If certain data was missing [52,53,54], we contacted the respective author for further details. Data of Devin et al. (2016) [54] was received and included into the analysis. If specific data was not presented numerically [52, 53], we extracted values from a figure by using the WebPlotDigitizer Tool [55]. Due to different ways of presenting and analyzing results, studies with seemingly identical samples were still included (Table 1) [46, 49, 56,57,58]. To categorize interval durations, we set three groups, i.e., ≤1min, 1–3min, and ≥3min, based on the diversity of the available data.
Data synthesis and analysis
Only studies that analyzed a direct comparison of MICT and HIIT were included in the meta-analysis. Statistics, forest plot, and funnel plot were realized using RevMan (Review Manager Version 5.4, The Cochrane Collaboration, 2020) and IBM SPSS Statistics 29. Figure 4 was created using Grapher 12 (Golden Software). As all outcome measures were continuous variables, the intervention effects of each RCT were calculated using the standardized mean difference (SMD). A funnel plot was created to analyze symmetry and distribution for possible publication bias [59].
As the included RCTs differed in several aspects, the pooled effect size was calculated using the random-effects model, which is generally recommended [60] when heterogeneity between studies is assumed. The effect size of the change in V̇O2PEAK/V̇O2MAX (SMD) was calculated using the following equation [61]:
x 1 and x2 are the sample means in the two groups [61]. The guideline values proposed by Cohen for the interpretation of the SMD are small (0.2), medium (0.5), and large (0.8) [62].
Heterogeneity between the included studies was assessed using the chi-square test and the I2 statistic. The I2 statistic determines the percentage of variability in the effect estimates that can be attributed to heterogeneity and can be interpreted as follows: 0–30% represents low heterogeneity, 30–60% represents moderate heterogeneity, and 60–100% represents high heterogeneity [63]. By pooling the SD values, a more accurate estimate of their joint value was obtained. SDPOOL was based on the SD from the baseline and the post values of the intervention group.
n 1 and n2 are sample sizes of each group, whereas S1 and S2 are the standard deviations in the two groups. In some cases (e.g., [52]), no SD was available for pre- and/or post-values. Standard deviations were consequently calculated using the standard error (SE) or confidence interval (CI):
Dividing the upper and lower limit of the CI by 3.92 was only used when a normal distribution could be assumed (e.g., large sample size) or was specifically reported. Otherwise, this value was based on a t-distribution (degrees of freedom −1, α = 0.05, two-tailed) [64].
The SE of the SMD is the square root of the variance (VD) of the SMD [61]:
Furthermore, we conducted a correlation analysis between the change in V̇O2PEAK/V̇O2MAX (% change, effect size (ES)) and the total exercise volume within the intervention group (HIIT).
Due to a violation of the assumption of normal distribution, a rank correlation (Spearman’s rho (ρ)) was used and interpreted according to Cohen [62]. As a positive correlation between the number of training sessions and V̇O2PEAK was already shown [65], we hypothesized an improvement in V̇O2PEAK/V̇O2MAX and therefore performed a one-tailed correlation test. Statistical significance was assumed at p<0.05.
Study quality and risk of bias assessment
Study quality was assessed using the Tool for the assEssment of Study qualiTy and reporting in EXercise (TESTEX). TESTEX is a 15-point scale and includes 5 points for study quality and 10 points for study reporting. This assessment tool was specifically designed for use in exercise training studies. A high total score indicates high study quality [66]. FITT and TESTEX have already been used in the context of cancer and exercise (e.g., [67]).
In addition, the revised Cochrane risk-of-bias tool [68] for randomized trials was used interdependently by two assessors to assess risk of bias. Five domains each relate to different aspects of bias: 1, randomization process; 2, deviations from the intended interventions; 3, missing outcome data; 4, outcome measurement; 5, selection of the reported outcome. To support the assessment of bias risk for the domain, the respective signal questions were answered and algorithms were followed to link the answers to the signal questions with suggestions for the resulting bias risk assessment [69]. The evaluation results of the publications consulted and evaluated for this work are shown (Fig. 2).
Results
The literature search resulted in a total of 584 records. A total of 209 titles were not included due to duplication. The remaining 372 titles were screened with regard to title and abstract. After further exclusions, 31 publications were finally included in the review. Due to the lack of adequate study data, no meta-analysis could be performed regarding the functional outcome. Eight studies were included in the meta-analysis of relV̇O2PEAK (Fig. 1).
The studies achieved an average total score of 11.6±1.3 (9–14) on the TESTEX scale. The average study quality was assessed high, with 4.0±0.9 (2–5) points, the study reporting dimension was moderately high, with 7.6±1.2 (5–9) points (see Supplementary Table). Therefore, no study had to be excluded due to poor study quality.
Based on the risk of bias analysis, the included studies were not found to be high risk. Only domain 5 “Selection of the reported result” was ranked having “some concerns,” as the relevant information could not be obtained from the available publications (Fig. 2).
Study population
A total of n=1555 patients aged 57.4±8.6 years were included in the review. A total of n=268 patients aged 59.11±5.11 years were included in the meta-analysis. Cohorts with different cancer types (e.g., [71]) or a specific indication (e.g., prostate cancer [72]) were studied. The population varied between 16 and 151 cancer patients. The patients were at different diagnostic stages of cancer (UICC stages I–IV). When participating in the respective interventions, 8 years [46, 49], 6 years [73], 4.1 years [74], 1–2 years [75], and ≤ years [71, 76, 77] elapsed after cancer diagnosis, respectively. In some cases, training was used preoperatively (e.g., cystectomy, lung resection, liver resection) [44, 56,57,58, 78, 79]. In two studies, training was performed in-treatment, concomitantly to usual care, such as chemotherapy [45] or epidermal growth factor receptor inhibitor therapy [80]. In one study, patients participated in the HIIT intervention within the aftercare period shortly after completing chemotherapy and radiotherapy [81].
Study design
Twenty-three of the selected publications comprised a two-armed design. Eight studies included a three-armed design [52,53,54, 71, 73,74,75,76,77, 82]. Patients were assigned to an intervention group (HIIT) or a control group (e.g., UC, MICT). Twenty-four studies compared HIIT with UC [44,45,46, 49, 52, 56,57,58, 65, 71,72,73, 77,78,79,80,81,82,83,84,85,86,87,88], 9 compared HIIT with MICT [52,53,54, 71, 73,74,75,76,77, 82, 89].
Functional performance
Functional outcomes were assessed in 12 studies [53, 56, 58, 71, 72, 76, 77, 83,84,85,86,87]. All of these included the 6MWT, either as the only functional assessment or in combination with others. Significant improvement after HIIT was shown in seven cases [56, 58, 71, 72, 76, 83, 85], six of which documented improved walking distance and one of which had a significant positive change in mobility [72]. Functional performance based on walking distance (6MWT) increased by 8.63±6.91% (range 1.73 to 19.02%) after HIIT and by 4.61±3.88% after the continuous method. Two studies analyzed the increase in maximal walking distance and additionally compared HIIT and MICT: The results show a distinct superiority of the respective HIIT group (+18.53% HIIT vs. +1.16% MICT [76]; +19.02% HIIT vs. +7.35% MICT [71]). No further meta-analytic approaches could be derived.
Physical performance and meta-analysis
RelV̇O2PEAK and V̇O2MAX were assessed as the outcome in 24 [44, 46, 48, 49, 52,53,54, 56,57,58, 65, 72,73,74,75, 77,78,79,80, 82, 84, 87,88,89] and 2 [45, 81] studies, respectively (Table 1). HIIT increased relV̇O2PEAK by 10.68±6.48%, while MICT led to improvements of 7.40±4.29% (range −0.37 to 22.41%). A significant improvement after HIIT was shown either in an improvement in the relV̇O2PEAK or V̇O2MAX from pretest to posttest [44, 46, 49, 54, 56,57,58, 73, 74, 77, 79,80,81]. Several times the respective between group differences showed a statistically significant improvement after HIIT, compared to UC [46, 49, 57, 58, 72, 79,80,81, 83,84,85] (Table 1).
Eight studies were analyzed for the effects of MICT versus HIIT on the increase in oxygen uptake [52,53,54, 74, 75, 77, 82, 89]. The meta-analysis showed that HIIT had a small but significant main effect (SMD=0.37; CI 0.09–0.65; p=0.009). One study showed a preference toward the MICT method [75], while another [73] did not favor either form of training. The heterogeneity between the studies was I2 = 0% (Fig. 3A). Due to its symmetry and data distribution, the funnel plot indicates no strong publication bias (Fig. 3B). Dolan et al. [73] had to be excluded from the meta-analysis because of missing baseline data (values were only presented for the entire population). Therefore, no specific effects for either HIIT or MICT could be calculated.
Training protocol and parameters
The duration of the training intervention was 8.0±3.6 weeks and varied between 2.5 and 12 weeks. Patients completed a mean of 3.0±0.4 training sessions per week (Table 1).
The 31 publications differed regarding the duration of intervals and rest, or different ratios of loading and unloading durations. The analyzed studies used interval durations between 0.25 and 5 minutes. The average interval length was 1.9±1.6 min. Seventeen studies implemented an interval of ≤1min [44, 45, 48, 52, 56,57,58, 65, 71, 75,76,77, 82,83,84,85,86], 10 studies used intervals of ≥3min duration per interval [46, 49, 54, 73, 74, 78, 80, 81, 88, 89], and three studies used intervals between 1 and 3min [53, 72, 87]. The duration of MICT (excluding warm up and cool down) was 31.50±15.64min (20–60min). Only one study reported a distance in miles [73]. The intervals were repeated 9.5±10.6 times (range 4–40 times). Two studies reported no information on specific interval design [79, 80]. The correlation between effect sizes regarding relV̇O2PEAK showed a significant moderate relationship (Spearman’s ρ=0.49; p=0.03) with total training volume, whereas percentage increase showed no significant correlation (Spearman’s ρ=0.24; p=0.14). If Kang et al. [74] is excluded from the correlation analysis as an inconsistent outlier due to an exceptional low effect while applying a high total training volume, we can state a moderate to high correlation (Spearman’s ρ=0.75; p<0.01) for the remaining 15 studies. Due to missing data (e.g., missing SD), it was not possible to calculate SDPOOL for every study. Finally, we calculated 22 data points for percentage change relV̇O2PEAK [44, 46, 48, 52,53,54, 57, 65, 72,73,74,75, 77,78,79,80,81,82, 84, 87,88,89] and 16 data points for ESPOOL [46, 48, 52,53,54, 57, 65, 72, 74, 75, 78,79,80, 82, 84, 88] (Fig. 4).
Rank correlation (Spearman’s rho (ρ)) between relV̇O2PEAK and total exercise volume (dots and solid line) as well as rank correlation between ESPOOL of pre to post values of relV̇O2PEAK and total exercise volume (triangles and dashed line); total exercise volume = training weeks × training frequency per week × duration of one session; note: only 15 triangles are visible due to overlay
In addition, different training intensities were applied. Nine of the 31 included publications selected power-related intensity parameters, for example 100–120% of the maximum power achieved during the pre-exercise cardiopulmonary exercise test (CPET baseline) or 90% peak power output [44, 45, 53, 56,57,58, 65, 77, 83]. Five of the 31 included studies used intensities based on V̇O2 values achieved during CPET and trained at 75–95% relV̇O2PEAK [46, 49, 72, 73, 79]. Fourteen studies used 85–95% of maximum heart rate (HRMAX) as the exercise intensity in the HIIT intervention groups [48, 52, 54, 71, 74,75,76, 81, 82, 85,86,87,88,89]. Further studies used subjectively perceived exertion [78, 84], VO2PEAK, or subjectively perceived exertion [80].
HIIT was performed on the cycle ergometer (n=20) or treadmill (n=5), a combination of cycling ergometers or treadmills (n=2), using body weight exercises (n=1), training outdoors (n=1), or a free selection of multiple devices (n=1) (Table 1). One study did not provide any specific information [73].
Schmitt et al. [75] organized the training outside on an uphill road with short walking breaks between intervals. The interval training in Dolan et al. [73] started with low-intensity intervals (65–75% relV̇O2PEAK) for the first 2 weeks and increased progressively to 80–95% relV̇O2PEAK. As the intensity increased, the duration of the intervals decreased from 4 to 2 min.
Discussion
The aim of this systematic review was to determine the effects of HIIT on the functional outcome and relV̇O2PEAK in cancer patients. In addition, a meta-analytic approach compares HIIT vs. MICT regarding relV̇O2PEAK. We also provide a detailed overview of implemented training parameters.
Functional outcomes
The functional outcomes walking distance (6MWT), mobility (SRT), grip strength (GS), and lower extremity strength (STS) were found in the reviewed studies. The data at hand suggest that an intervention with HIIT can significantly improve walking distance [56, 58, 71, 76, 83, 85] and mobility [72] in cancer patients. When HIIT was performed with strength-oriented body weight exercises, grip strength improved twice as much compared to UC [84]. This indicates that the method and manner HIIT is implemented may determine possible functional outcomes. It is plausible that GS would not be altered after HIIT, riding a stationary bike where no specific GS is required. In some cases, functional parameters such as TUG and STS remained almost unchanged following HIIT so that no differences from the control group can be observed [72, 83]. Toohey et al. [71, 76] depicted opposing results for STS in a comparison between HIIT and MICT: In one study, the MICT group showed stronger improvements [76] whereas the another study [71] presents a stronger effect after HIIT.
The resulting walking distance can be used as a marker of aerobic fitness and as a protective factor for cancer mortality [90] and therefore is of great relevance. Accordingly, it is particularly significant for cancer patients to achieve practically relevant improvements in walking distance as a benefit of HIIT. Walking distance is also related to health status in cancer patients (quality of life, cancer-related symptoms) [91]. In addition, the 6MWT could be used to plan and control training intensities, for example in the context of HIIT [92]. The observed improvements [56, 58, 71, 72, 76, 83,84,85,86] exceeded the minimal clinically meaningful difference for multiple patient groups (including cancer survivors) of 14–30.5m [93] or 62.5m [94] improvements, respectively. Nevertheless, contrary results were also found for walking distance, HIIT resulted in marginal gains [87], and MICT resulted in greater improvement [53].
Due to lack of a specific comparison between HIIT and MICT, no meta-analysis for functional outcomes could be performed. Toohey et al. [71, 76] presented 6MWT data for both training protocols that suggested a superiority of HIIT. We found more 6MWT [53, 56, 58, 72, 83,84,85,86,87] and other functional outcomes [71, 72, 76, 83, 84], but none of these studies compared HIIT vs. MICT. Although specific evidences for cancer patients are limited, our meta-analytic data are in line with found differences between HIIT and MICT in healthy elder populations: Coswig et al. [38] found greater improvements in STS and 6MWT after HIIT (vs. MICT) in elderly women. Coetsee and Terblanche [39] presented greater functional improvements in TUG after HIIT (vs. MICT) in a healthy older population. Our results suggest that HIIT has the same tendency to improve functional performance, but further studies need to address the direct comparison between HIIT and MICT to verify these findings.
Physical outcomes
The review of all studies showed a rather clear superiority of complementary HIIT compared to UC [46, 49, 57, 58, 72, 79,80,81, 83,84,85]. Control groups receiving usual care alone showed a partial decrease in relV̇O2PEAK over the intervention period [46, 52, 57, 58, 72, 73, 78,79,80, 82, 84, 88]. We found that both HIIT and MICT were shown to improve physical performance in patients across all cancer stages I–IV. Effects with HIIT occurred despite different training protocols (intervention duration, training frequency, training volume, or training intensity).
As shown in the meta-analysis, 7 out of 8 studies are presenting pronounced results after HIIT in terms of relV̇O2PEAK (SMD 0.37; 95% CI 0.09 to 0.65; I2=0%; p=0.01); we can conclude superiority of the HIIT modality vs. MICT (Fig. 3). Mugele et al. [42] found no clear superiority of HIIT compared to MICT for relV̇O2PEAK (MD 1.36; 95% CI −1.62 to 4.35; p=0.37). Due to a greater data source to evaluate this comparison, we conclude that HIIT may be more beneficial that MICT in order to improve relV̇O2PEAK. Hooshmand-Moghadam et al. [52] also concluded that HIIT is more beneficial than MICT for improving physical fitness (here: relV̇O2PEAK + low body strength).
Different methods to average peak values (e.g., over 20s or 30s) were used to determine relV̇O2PEAK/V̇O2MAX, which limits the direct comparability of the data [95,96,97,98,99]. The extent to which V̇O2MAX can be achieved with in patients is debated [100, 101]. In many cases, a symptom-limited V̇O2PEAK is assumed to be lower than the actual V̇O2MAX [100]. Most studies included in this review reported the relV̇O2peak. Of the reviews studies, only Alizadeh et al. [81] and Lee et al. [45] acclaimed having achieved a V̇O2MAX. Alizadeh et al. [81] estimated the V̇O2MAX using a submaximal test (Rockport 1 mile walk test) while Lee et al. [45] determined V̇O2MAX using a ramp test on a cycling ergometer but did not provide information on criteria for workload. In general, when leveling off is reached, it is assumed that exhaustion and V̇O2MAX are reached [98, 100, 101]. Thus, it should be taken into account that, if necessary, patients did not reach a leveling off and relV̇O2PEAK values were collected here.
When interpreting the aforementioned results, it should be noted that they only indirectly reflect the effects for the individual. It is therefore possible that the HIIT training protocol can achieve significantly higher, but also lower functional or physical effects in individual cases. Partially contradictory results are shown, for example, in the study by Boereboom et al. [102], in which individuals show strong positive changes in oxygen uptake, while others show negative changes. A clear attribution of cause (e.g., dependence on baseline level, number of training sessions performed) was not given. It is plausible that novice or, as in this case, deconditioned patients show significantly higher individual training effects than a person experienced in training [103]. In some cases, novices without experience with intensive training [46, 49] or inactive patients (did not achieve guideline recommendations for moderate or intensive activity) were explicitly included [71, 78].
Training parameters and implementation
We stated that HIIT was performed on both the treadmill [46, 49, 72, 76, 80, 81, 88] or the cycling ergometer [44, 45, 48, 52,53,54, 56,57,58, 65, 71, 74, 76,77,78,79,80, 82, 83, 85, 86, 89]. In two cases, training was performed by walking outdoors [73, 75]. Since running promotes greater muscle mass than cycling, the working muscle mass used differs between the “running” and “cycling” forms of exercise, limiting a direct comparison [104]. HIIT on the treadmill was shown to result in higher heart rate and oxygen uptake than the same exercise on the cycling ergometer in healthy individuals [105]. It is possible that the high-intensity loads outside (weather, ground conditions, elevation profile) compared to controlled laboratory conditions (treadmill, cycling ergometer) may have an impact on the target parameters. Two studies differed by applying home-based HIIT: Exercises were performed outside or at local training resources in various forms of endurance training [87] or using the patients’ own body weight [84].
A respective HIIT design was approached and implemented differently by the authors of each study. Thus, interval durations varied from 0.25 to 5 min (mean 2.2±1.8 min) and were repeated 4–40 times (mean 12±13.7). Furthermore, it is important to question the extent to which the load design of 4×4min [46, 49, 81] or 6×5min [78] still fulfills the characteristics of short, high-intensity intervals of HIIT. In this review, we observed a differing values to measure training intensity (%HFMAX, % relV̇O2PEAK) (Table 1). Other studies used other data (e.g., % peak power output). Intensities ≤80% were documented that, according to the definition by MacInnis et al. [106] (≥80% HRMAX, mostly 85–95% HRMAX), they did not correspond to the definitions of HIIT (e.g., 90). In some cases, ranges of ≤80 to ≥80% were also reported [46, 49, 73, 78, 88] (Table 1).
Schlüter et al. [107] compared 10×1min vs. 4×4min HIIT acutely protocols at 85–95% HRMAX (breast and prostate cancer patients) and concluded that a 4×4min protocol induced a higher energy expenditure and higher cardio-circulatory and metabolic strain. Therefore, if a high training stimulus is intended, a longer interval duration is preferable. However, an instructing physical therapist has to supervise if the patients tolerate rather long intervals, especially when undergoing therapy. Low training experience could also be a limiting factor in order to maintain intense intervals for several minutes.
A meta-analysis by Bacon et al. [108] showed that the design of the load factors during HIIT has an influence on the results in healthy individuals. It seems that especially the duration of the intervention in weeks is decisive. To take that into account, we included this parameter in total training volume.
A major criteria and possible promise of HIIT (compared with MICT) is generating relevant effects in a short time through short, intense intervals. Therefore, we analyzed the correlations between effects of HIIT on relV̇O2PEAK and total training volume (Fig. 4). We are aware of possible confounding factors that have been considered with regard to the reliability of this statement, yet we selected a specific training parameter directly in the context of HIIT. The analysis indicates no direct dependence of total training volume and effects on relV̇O2PEAK (Fig. 4). HIIT appears to be suitable for cancer patients to achieve relevant effects on endurance performance even in a short but intensive training period, although Lavín-Pérez et al. [109] point out that the exercise level should be at least 8 weeks, 2×/week (of which 15min HIIT/week) in cancer patients to achieve the highest return in health-related quality of life. In addition to intensity, it is possible that the total training volume represents a decisive parameter for training management of HIIT in cancer patients.
Yet, every implementation of HIIT has to be depending on the individual physical capabilities which may be altered due to timing during therapy or aftercare respectively. Even though the data indicate that HIIT is beneficial and helpful in improving performance, it still represents an intensive form of endurance exercise, where the patient’s health condition has a limiting effect on its applicability. A patient undergoing treatment may suffer from side effects, while the performance in aftercare may be impaired due to long-term cancer therapy and management. An individualized and supervised training regimen, in which specific training parameters can be modulated, could be key to implementing HIIT in prehabilitation, during treatment and aftercare as well. There is no specific red flag that excludes HIIT in any stage. A regression analysis of the influence of training volume and intensity could not be performed due to the partially imprecise or missing indications of the achieved training intensity (ranges from-to) (Table 1). As mentioned earlier, the study by Dolan et al. [73] was included even though the authors chose a progressive increase in intensity. This should be taken into account when interpreting the results and could be one reason why interval training was not superior to the continuous method in terms of relV̇O2PEAK improvement, in contrast to other studies.
Based on this experimental application of the HIIT training form, insights for further therapeutic practice can be derived and aspects of the suitability and practical implementation of HIIT can be specified. In the context of endurance training in cancer patients, interval training can be used as a suitable, tolerable form of exercise. This is especially true, if a continuous load without breaks and over a longer period of time is not yet tolerated. HIIT is a suitable form of endurance training to improve cancer-related fatigue [49, 110]. Taking into account the shorter “economic” training time, HIIT may be sufficient to contribute to the prevention of cardiovascular events or the reduction of cancer related fatigue. HIIT may thus represent an important contribution to improving physical fitness and health-related outcomes, and may add significant value compared to usual care [42].
Summary and outlook
The review showed that different functional outcomes were positively altered through HIIT. Our data indicates that HIIT might be more effective than MICT. Because functional outcomes were often not considered in the reviewed HIIT studies, no meta-analytic approach could be realized regarding the functional outcomes. We suggest that more attention should be paid to the functional outcome component to enable further direct comparisons between HIIT and MICT in terms of outcomes that are highly relevant to the daily lives of cancer survivors.
Furthermore, this review showed that positive changes in relV̇O2PEAK were achieved with both MICT and HIIT, with HIIT usually having greater effects. Usual care alone mostly led to a decrease in performance. Results of the meta-analysis showed that HIIT appears to have greater effects on relV̇O2PEAK compared to MICT. Further studies are needed to verify these results for relV̇O2PEAK.
Precise information on frequency, duration, and intensity of the respective intervals cannot yet be given but could be optimized by the respective trainer in the future. Distinct relationships with various exercise factors (e.g., duration, intensity, frequency) have to be addressed in a targeted and systematic manner. Furthermore, the application of HIIT in the real clinical setting of cancer therapy should be verified. The present “black box” about how HIIT is implemented should be analyzed with concrete application-related data from clinical practice.
References
Dimeo FC (2011) Bedeutung von Sport in der onkologischen Akutbehandlung. Forum 26:31–33. https://doi.org/10.1007/s12312-011-0607-5
Friedenreich CM, Stone CR, Cheung WY, Hayes SC (2020) Physical activity and mortality in cancer survivors: a systematic review and meta-analysis. JNCI Cancer Spectr 4:pkz080. https://doi.org/10.1093/jncics/pkz080
McTiernan A, Friedenreich CM, Katzmarzyk PT et al (2019) Physical activity in cancer prevention and survival: a systematic review. Med Sci Sports Exerc 51:1252
Patel AV, Friedenreich CM, Moore SC et al (2019) American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc 51:2391
Caspersen CJ, Powell KE, Christenson GM (1985) Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 100:126–131
Pedersen BK, Saltin B (2015) Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 25(Suppl 3):1–72. https://doi.org/10.1111/sms.12581
Newton RU, Galvão DA (2008) Exercise in prevention and management of cancer. Curr Treat Options Oncol 9:135–146. https://doi.org/10.1007/s11864-008-0065-1
Silver JK (2015) Cancer prehabilitation and its role in improving health outcomes and reducing health care costs. Semin Oncol Nurs 31:13–30. https://doi.org/10.1016/j.soncn.2014.11.003
Cormie P, Zopf EM, Zhang X, Schmitz KH (2017) The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol Rev 39:71–92. https://doi.org/10.1093/epirev/mxx007
Wirtz P, Tomanek A, Baumann FT (2019) Bedeutung von Sport und Bewegung für cancer survivors. Forum 34:35–38. https://doi.org/10.1007/s12312-018-0541-x
Ibrahim EM, Al-Homaidh A (2011) Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol 28:753–765. https://doi.org/10.1007/s12032-010-9536-x
Serdà i Ferrer BC, van Roekel E, Lynch BM (2018) The role of physical activity in managing fatigue in cancer survivors. Curr Nutr Rep 7:59–69. https://doi.org/10.1007/s13668-018-0234-1
Romero SAD, Jones L, Bauml JM, Li QS, Cohen RB, Mao JJ (2018) The association between fatigue and pain symptoms and decreased physical activity after cancer. Support Care Cancer 26:3423–3430. https://doi.org/10.1007/s00520-018-4203-4
Koene RJ, Prizment AE, Blaes A, Konety SH (2016) Shared risk factors in cardiovascular disease and cancer. Circulation 133:1104–1114. https://doi.org/10.1161/CIRCULATIONAHA.115.020406
Jones LW, Habel LA, Weltzien E et al (2016) Exercise and risk of cardiovascular events in women with nonmetastatic breast cancer. J Clin Oncol 34:2743–2749. https://doi.org/10.1200/JCO.2015.65.6603
Vardar Yağlı N, Şener G, Arıkan H et al (2015) Do yoga and aerobic exercise training have impact on functional capacity, fatigue, peripheral muscle strength, and quality of life in breast cancer survivors? Integr Cancer Ther 14:125–132. https://doi.org/10.1177/1534735414565699
Scott JM, Zabor EC, Schwitzer E et al (2018) Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol 36:2297–2305. https://doi.org/10.1200/JCO.2017.77.5809
Jones LW, Liang Y, Pituskin EN et al (2011) Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist 16:112–120. https://doi.org/10.1634/theoncologist.2010-0197
Fuller JT, Hartland MC, Maloney LT, Davison K (2018) Therapeutic effects of aerobic and resistance exercises for cancer survivors: a systematic review of meta-analyses of clinical trials. Br J Sports Med 52:1311. https://doi.org/10.1136/bjsports-2017-098285
Schmid D, Leitzmann MF (2015) Cardiorespiratory fitness as predictor of cancer mortality: a systematic review and meta-analysis. Ann Oncol 26:272–278. https://doi.org/10.1093/annonc/mdu250
Dimeo FC, Thiel E (2008) Körperliche Aktivität und Sport bei Krebspatienten. Onkologe 14:31–37. https://doi.org/10.1007/s00761-007-1288-7
Hanakam F, Ferrauti A (2020) Ausdauertraining. In: Ferrauti A, Fett J, Frytz A (eds) Trainingswissenschaft für die Sportpraxis. Lehrbuch für Studium, Ausbildung und Unterricht im Sport. Springer Spektrum, Berlin, Heidelberg, pp 345–404
Gibala MJ, McGee SL (2008) Metabolic adaptations to short-term high-intensity interval training: a little pain for a lot of gain? Exerc Sport Sci Rev 36:58–63. https://doi.org/10.1097/JES.0b013e318168ec1f
Kessler HS, Sisson SB, Short KR (2012) The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med 42:489–509. https://doi.org/10.2165/11630910-000000000-00000
Alvarez C, Ramirez-Campillo R, Martinez-Salazar C et al (2016) Low-volume high-intensity interval training as a therapy for type 2 diabetes. Int J Sports Med 37:723–729. https://doi.org/10.1055/s-0042-104935
Choi H-Y, Han H-J, Choi J-W, Jung H-Y, Joa K-L (2018) Superior effects of high-intensity interval training compared to conventional therapy on cardiovascular and psychological aspects in myocardial infarction. Ann Rehabil Med 42:145–153. https://doi.org/10.5535/arm.2018.42.1.145
Meßler CF, Holmberg H-C, Sperlich B (2018) Multimodal therapy involving high-intensity interval training improves the physical fitness, motor skills, social behavior, and quality of life of boys with ADHD: a randomized controlled study. J Atten Disord 22:806–812. https://doi.org/10.1177/1087054716636936
Milanović Z, Sporiš G, Weston M (2015) Effectiveness of High-Intensity Interval Training (HIT) and continuous endurance training for VO2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med 45:1469–1481. https://doi.org/10.1007/s40279-015-0365-0
Ross LM, Porter RR, Durstine JL (2016) High-intensity interval training (HIIT) for patients with chronic diseases. J Sport Health Sci 5:139–144. https://doi.org/10.1016/j.jshs.2016.04.005
Batacan RB, Duncan MJ, Dalbo VJ, Tucker PS, Fenning AS (2017) Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. Br J Sports Med 51:494–503. https://doi.org/10.1136/bjsports-2015-095841
Wang K, Zhu Y, Wong SH-S et al (2021) Effects and dose-response relationship of high-intensity interval training on cardiorespiratory fitness in overweight and obese adults: a systematic review and meta-analysis. J Sports Sci 39:2829–2846. https://doi.org/10.1080/02640414.2021.1964800
Liou K, Ho S, Fildes J, Ooi S-Y (2016) High intensity interval versus moderate intensity continuous training in patients with coronary artery disease: a meta-analysis of physiological and clinical parameters. Heart Lung Circ 25:166–174. https://doi.org/10.1016/j.hlc.2015.06.828
But-Hadzic J, Dervisevic M, Karpljuk D et al (2021) Six-minute walk distance in breast cancer survivors-a systematic review with meta-analysis. Int J Environ Res Public Health 18. https://doi.org/10.3390/ijerph18052591
Kubo Y, Naito T, Mori K, Osawa G, Aruga E (2017) Skeletal muscle loss and prognosis of breast cancer patients. Support Care Cancer 25:2221–2227. https://doi.org/10.1007/s00520-017-3628-5
Neil-Sztramko SE, Kirkham AA, Hung SH, Niksirat N, Nishikawa K, Campbell KL (2014) Aerobic capacity and upper limb strength are reduced in women diagnosed with breast cancer: a systematic review. J Physiother 60:189–200. https://doi.org/10.1016/j.jphys.2014.09.005
Ten Tusscher MR, Groen WG, Geleijn E et al (2019) Physical problems, functional limitations, and preferences for physical therapist-guided exercise programs among Dutch patients with metastatic breast cancer: a mixed methods study. Support Care Cancer 27:3061–3070. https://doi.org/10.1007/s00520-018-4619-x
Grusdat NP, Stäuber A, Tolkmitt M et al (2022) Routine cancer treatments and their impact on physical function, symptoms of cancer-related fatigue, anxiety, and depression. Support Care Cancer 30:3733–3744. https://doi.org/10.1007/s00520-021-06787-5
Coswig VS, Barbalho M, Raiol R, Del Vecchio FB, Ramirez-Campillo R, Gentil P (2020) Effects of high vs moderate-intensity intermittent training on functionality, resting heart rate and blood pressure of elderly women. J Transl Med 18:88. https://doi.org/10.1186/s12967-020-02261-8
Coetsee C, Terblanche E (2017) The effect of three different exercise training modalities on cognitive and physical function in a healthy older population. Eur Rev Aging Phys Act 14:13. https://doi.org/10.1186/s11556-017-0183-5
Schmitt J (2019) Hoch-intensives Intervalltraining in der onkologischen Rehabilitation – Erfahrungen aus der Therapiepraxis. B&G Bewegungstherapie und Gesundheitssport 35:105–109. https://doi.org/10.1055/a-0860-1986
Palma S, Hasenoehrl T, Jordakieva G, Ramazanova D, Crevenna R (2021) High-intensity interval training in the prehabilitation of cancer patients-a systematic review and meta-analysis. Support Care Cancer 29:1781–1794. https://doi.org/10.1007/s00520-020-05834-x
Mugele H, Freitag N, Wilhelmi J et al (2019) High-intensity interval training in the therapy and aftercare of cancer patients: a systematic review with meta-analysis. J Cancer Surviv 13:205–223. https://doi.org/10.1007/s11764-019-00743-3
Gillen JB, Gibala MJ (2014) Is high-intensity interval training a time-efficient exercise strategy to improve health and fitness? Applied physiology, nutrition, and metabolism. Physiol Appl Nutr Metab 39:409–412. https://doi.org/10.1139/apnm-2013-0187
Blackwell JEM, Doleman B, Boereboom CL et al (2020) High-intensity interval training produces a significant improvement in fitness in less than 31 days before surgery for urological cancer: a randomised control trial. Prostate Cancer Prostatic Dis 23:696–704. https://doi.org/10.1038/s41391-020-0219-1
Lee K, Kang I, Mack WJ et al (2019) Feasibility of high intensity interval training in patients with breast cancer undergoing anthracycline chemotherapy: a randomized pilot trial. BMC Cancer 19:653. https://doi.org/10.1186/s12885-019-5887-7
Adams SC, DeLorey DS, Davenport MH et al (2017) Effects of high-intensity aerobic interval training on cardiovascular disease risk in testicular cancer survivors: a phase 2 randomized controlled trial. Cancer 123:4057–4065. https://doi.org/10.1002/cncr.30859
Morielli AR, Boule NG, Usmani N et al (2021) Effects of exercise during and after neoadjuvant chemoradiation on symptom burden and quality of life in rectal cancer patients: a phase II randomized controlled trial. J Cancer Surviv. https://doi.org/10.1007/s11764-021-01149-w
Reljic D, Herrmann HJ, Jakobs B et al (2022) Feasibility, safety, and preliminary efficacy of very low-volume interval training in advanced cancer patients. Med Sci Sports Exerc 54:1817–1830. https://doi.org/10.1249/MSS.0000000000002989
Adams SC, DeLorey DS, Davenport MH, Fairey AS, North S, Courneya KS (2018) Effects of high-intensity interval training on fatigue and quality of life in testicular cancer survivors. Br J Cancer 118:1313–1321. https://doi.org/10.1038/s41416-018-0044-7
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical research ed) 339:b2535. https://doi.org/10.1136/bmj.b2535
Wiskemann J (2014) Krafttraining als Supportivtherapie in der Onkologie. Dtsch Z Sportmed 2014. https://doi.org/10.5960/dzsm.2013.090
Hooshmand Moghadam B, Golestani F, Bagheri R et al (2021) The effects of high-intensity interval training vs. moderate-intensity continuous training on inflammatory markers, body composition, and physical fitness in overweight/obese survivors of breast cancer: a randomized controlled clinical trial. Cancers 13. https://doi.org/10.3390/cancers13174386
Minnella EM, Ferreira V, Awasthi R et al (2020) Effect of two different pre-operative exercise training regimens before colorectal surgery on functional capacity: a randomised controlled trial. Eur J Anaesthesiol 37:969–978. https://doi.org/10.1097/EJA.0000000000001215
Devin JL, Sax AT, Hughes GI et al (2016) The influence of high-intensity compared with moderate-intensity exercise training on cardiorespiratory fitness and body composition in colorectal cancer survivors: a randomised controlled trial. J Cancer Surviv 10:467–479. https://doi.org/10.1007/s11764-015-0490-7
Rohatgi A. Webplotdigitizer: Version 4.6 2022.
Bhatia C, Kayser B (2019) Preoperative high-intensity interval training is effective and safe in deconditioned patients with lung cancer: a randomized clinical trial. J Rehabil Med 51:712–718. https://doi.org/10.2340/16501977-2592
Karenovics W, Licker M, Ellenberger C et al (2017) Short-term preoperative exercise therapy does not improve long-term outcome after lung cancer surgery: a randomized controlled study. Eur J Cardiothorac Surg 52:47–54. https://doi.org/10.1093/ejcts/ezx030
Licker M, Karenovics W, Diaper J et al (2017) Short-term preoperative high-intensity interval training in patients awaiting lung cancer surgery: a randomized controlled trial. J Thorac Oncol 12:323–333. https://doi.org/10.1016/j.jtho.2016.09.125
Sterne JA, Gavaghan D, Egger M (2000) Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 53:1119–1129. https://doi.org/10.1016/S0895-4356(00)00242-0
Gliner JA, Morgan GA, Harmon RJ (2003) Pretest-posttest comparison group designs: analysis and interpretation. J Am Acad Child Adolesc Psychiatry 42:500–503. https://doi.org/10.1097/01.CHI.0000046809.95464.BE
Borenstein M, Hedges LV, Higgins JP, Rothstein HR (2009) Introduction to meta-analysis. Wiley, Chichester
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Hillsdale, NJ
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
Higgins J, Li T, Deeks J (2022) Chapter 6: choosing effect measures and computing estimates of effect. In: Higgins J, Thomas J, Chandler J et al (eds) Cochrane Handbook for Systematic Reviews of Interventions version 6.3
Djurhuus SS, Simonsen C, Toft BG et al (2023) Exercise training to increase tumour natural killer-cell infiltration in men with localised prostate cancer: a randomised controlled trial. BJU Int 131:116–124. https://doi.org/10.1111/bju.15842
Smart NA, Waldron M, Ismail H et al (2015) Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Healthc 13:9–18. https://doi.org/10.1097/XEB.0000000000000020
Bjørke ACH, Sweegers MG, Buffart LM, Raastad T, Nygren P, Berntsen S (2019) Which exercise prescriptions optimize V̇O2 max during cancer treatment?-A systematic review and meta-analysis. Scand J Med Sci Sports 29:1274–1287. https://doi.org/10.1111/sms.13442
Higgins JPT, Altman DG, Gøtzsche PC et al (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed) 343:d5928. https://doi.org/10.1136/bmj.d5928
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed) 366:l4898. https://doi.org/10.1136/bmj.l4898
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Toohey K, Pumpa K, McKune A et al (2018) Does low volume high-intensity interval training elicit superior benefits to continuous low to moderate-intensity training in cancer survivors? World J Clin Oncol 9:1–12. https://doi.org/10.5306/wjco.v9.i1.1
Kang D-W, Fairey AS, Boulé NG, Field CJ, Wharton SA, Courneya KS (2021) Effects of exercise on cardiorespiratory fitness and biochemical progression in men with localized prostate cancer under active surveillance: the ERASE randomized clinical trial. JAMA Oncol 7:1487–1495. https://doi.org/10.1001/jamaoncol.2021.3067
Dolan LB, Campbell K, Gelmon K, Neil-Sztramko S, Holmes D, McKenzie DC (2016) Interval versus continuous aerobic exercise training in breast cancer survivors--a pilot RCT. Support Care Cancer 24:119–127. https://doi.org/10.1007/s00520-015-2749-y
Devin JL, Jenkins DG, Sax AT et al (2018) Cardiorespiratory fitness and body composition responses to different intensities and frequencies of exercise training in colorectal cancer survivors. Clin Colorectal Cancer 17:e269–e279. https://doi.org/10.1016/j.clcc.2018.01.004
Schmitt J, Lindner N, Reuss-Borst M, Holmberg H-C, Sperlich B (2016) A 3-week multimodal intervention involving high-intensity interval training in female cancer survivors: a randomized controlled trial. Physiol Rep 4. https://doi.org/10.14814/phy2.12693
Toohey K, Pumpa KL, Arnolda L et al (2016) A pilot study examining the effects of low-volume high-intensity interval training and continuous low to moderate intensity training on quality of life, functional capacity and cardiovascular risk factors in cancer survivors. PeerJ 4:e2613. https://doi.org/10.7717/peerj.2613
Toohey K, Pumpa K, McKune A et al (2020) The impact of high-intensity interval training exercise on breast cancer survivors: a pilot study to explore fitness, cardiac regulation and biomarkers of the stress systems. BMC Cancer 20:787. https://doi.org/10.1186/s12885-020-07295-1
Banerjee S, Manley K, Shaw B et al (2018) Vigorous intensity aerobic interval exercise in bladder cancer patients prior to radical cystectomy: a feasibility randomised controlled trial. Support Care Cancer 26:1515–1523. https://doi.org/10.1007/s00520-017-3991-2
Dunne DFJ, Jack S, Jones RP et al (2016) Randomized clinical trial of prehabilitation before planned liver resection. Br J Surg 103:504–512. https://doi.org/10.1002/bjs.10096
Hwang C-L, Yu C-J, Shih J-Y, Yang P-C, Wu Y-T (2012) Effects of exercise training on exercise capacity in patients with non-small cell lung cancer receiving targeted therapy. Support Care Cancer 20:3169–3177. https://doi.org/10.1007/s00520-012-1452-5
Alizadeh AM, Isanejad A, Sadighi S, Mardani M, kalaghchi B, Hassan ZM. (2019) High-intensity interval training can modulate the systemic inflammation and HSP70 in the breast cancer: a randomized control trial. J Cancer Res Clin Oncol 145:2583–2593. https://doi.org/10.1007/s00432-019-02996-y
Northey JM, Pumpa KL, Quinlan C et al (2019) Cognition in breast cancer survivors: a pilot study of interval and continuous exercise. J Sci Med Sport 22:580–585. https://doi.org/10.1016/j.jsams.2018.11.026
Lee K, Norris MK, Wang E, Dieli-Conwright CM (2021) Effect of high-intensity interval training on patient-reported outcomes and physical function in women with breast cancer receiving anthracycline-based chemotherapy. Support Care Cancer 29:6863–6870. https://doi.org/10.1007/s00520-021-06294-7
Ochi E, Tsuji K, Narisawa T et al (2022) Cardiorespiratory fitness in breast cancer survivors: a randomised controlled trial of home-based smartphone supported high intensity interval training. BMJ Support Palliat Care 12:33–37. https://doi.org/10.1136/bmjspcare-2021-003141
Piraux E, Caty G, Renard L et al (2021) Effects of high-intensity interval training compared with resistance training in prostate cancer patients undergoing radiotherapy: a randomized controlled trial. Prostate Cancer Prostatic Dis 24:156–165. https://doi.org/10.1038/s41391-020-0259-6
Piraux E, Reychler G, Vancraeynest D, Geets X, Léonard D, Caty G (2022) High-intensity aerobic interval training and resistance training are feasible in rectal cancer patients undergoing chemoradiotherapy: a feasibility randomized controlled study. Rep Pract Oncol Radiother 27:198–208. https://doi.org/10.5603/RPOR.a2022.0036
Wood WA, Weaver M, Smith-Ryan AE, Hanson ED, Shea TC, Battaglini CL (2020) Lessons learned from a pilot randomized clinical trial of home-based exercise prescription before allogeneic hematopoietic cell transplantation. Supportive Care Cancer 28:5291–5298. https://doi.org/10.1007/s00520-020-05369-1
Samhan AF, Ahmed AS, Mahmoud WS, Abdelhalim NM (2021) Effects of high-intensity interval training on cardiorespiratory fitness, body composition, and quality of life in overweight and obese survivors of breast cancer. Rehabil Oncol 39:168–174. https://doi.org/10.1097/01.REO.0000000000000270
Bell RA, Baldi JC, Jones LM (2021) Additional cardiovascular fitness when progressing from moderate- to high-intensity exercise training in previously trained breast cancer survivors. Support Care Cancer 29:6645–6650. https://doi.org/10.1007/s00520-021-06259-w
Burr JF, Bredin SSD, Faktor MD, Warburton DER (2011) The 6-minute walk test as a predictor of objectively measured aerobic fitness in healthy working-aged adults. Phys Sportsmed 39:133–139. https://doi.org/10.3810/psm.2011.05.1904
Galiano-Castillo N, Arroyo-Morales M, Ariza-Garcia A et al (2016) The six-minute walk test as a measure of health in breast cancer patients. J Aging Phys Act 24:508–515. https://doi.org/10.1123/japa.2015-0056
Tubiana-Mathieu N, Cornette T, Mandigout S et al (2021) Can the six-minute walk test be used to individualize physical activity intensity in patients with breast cancer? Cancers 13. https://doi.org/10.3390/cancers13225851
Bohannon RW, Crouch R (2017) Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract 23:377–381. https://doi.org/10.1111/jep.12629
Foley MP, Hasson SM (2016) Effects of a community-based multimodal exercise program on health-related physical fitness and physical function in breast cancer survivors: a pilot study. Integr Cancer Ther 15:446–454. https://doi.org/10.1177/1534735416639716
Cunha FA, Midgley AW, Monteiro WD, Farinatti PTV (2010) Influence of cardiopulmonary exercise testing protocol and resting VO(2) assessment on %HR(max), %HRR, %VO(2max) and %VO(2)R relationships. Int J Sports Med 31:319–326. https://doi.org/10.1055/s-0030-1248283
Keren G, Magazanik A, Epstein Y (1980) A comparison of various methods for the determination of VO2max. Eur J Appl Physiol 45:117–124. https://doi.org/10.1007/BF00421319
Kohrt WM, O'Connor JS, Skinner JS (1989) Longitudinal assessment of responses by triathletes to swimming, cycling, and running. Med Sci Sports Exerc 21:569–575
Meyer T, Kindermann W (1999) Die maximale sauerstoffaufnahme (VO2max). Dtsch Z Sportmed 50:285–286
Robergs RA, Dwyer D, Astorino T (2010) Recommendations for improved data processing from expired gas analysis indirect calorimetry. Sports Med 40:95–111. https://doi.org/10.2165/11319670-000000000-00000
Green S, Askew C (2018) V̇o2peak is an acceptable estimate of cardiorespiratory fitness but not V̇o2max. J Appl Physiol 125:229–232. https://doi.org/10.1152/japplphysiol.00850.2017
Poole DC, Jones AM (2017) Measurement of the maximum oxygen uptake V̇o2max: V̇o2peak is no longer acceptable. J Appl Physiol 122:997–1002. https://doi.org/10.1152/japplphysiol.01063.2016
Boereboom CL, Blackwell JEM, Williams JP, Phillips BE, Lund JN (2019) Short-term pre-operative high-intensity interval training does not improve fitness of colorectal cancer patients. Scand J Med Sci Sports 29:1383–1391. https://doi.org/10.1111/sms.13460
Boereboom CL, Phillips BE, Williams JP, Lund JN (2016) A 31-day time to surgery compliant exercise training programme improves aerobic health in the elderly. Tech Coloproctol 20:375–382. https://doi.org/10.1007/s10151-016-1455-1
Millet GP, Vleck VE, Bentley DJ (2009) Physiological differences between cycling and running: lessons from triathletes. Sports Med 39:179–206. https://doi.org/10.2165/00007256-200939030-00002
Twist C, Bott R, Highton J (2023) The physiological, perceptual and neuromuscular responses of team sport athletes to a running and cycling high intensity interval training session. Eur J Appl Physiol 123:113–120. https://doi.org/10.1007/s00421-022-05053-8
MacInnis MJ, Gibala MJ (2017) Physiological adaptations to interval training and the role of exercise intensity. J Physiol 595:2915–2930. https://doi.org/10.1113/JP273196
Schlüter K, Schneider J, Sprave T, Wiskemann J, Rosenberger F (2019) Feasibility of two high-intensity interval training protocols in cancer survivors. Med Sci Sports Exerc 51:2443–2450. https://doi.org/10.1249/MSS.0000000000002081
Bacon AP, Carter RE, Ogle EA, Joyner MJ (2013) VO2max trainability and high intensity interval training in humans: a meta-analysis. PloS One 8:e73182. https://doi.org/10.1371/journal.pone.0073182
Lavín-Pérez AM, Collado-Mateo D, Mayo X et al (2021) Effects of high-intensity training on the quality of life of cancer patients and survivors: a systematic review with meta-analysis. Sci Rep 11:15089. https://doi.org/10.1038/s41598-021-94476-y
Chang M, Wang J, Hashim HA, Xie S, Malik AA (2022) Effect of high-intensity interval training on aerobic capacity and fatigue among patients with prostate cancer: a meta-analysis. World J Surg Oncol 20:348. https://doi.org/10.1186/s12957-022-02807-8
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Literature research, data collection, and analysis were performed by Tilo Neuendorf, Ralf Haase, Sophia Schroeder, and Nico Nitzsche. The first draft of the manuscript was written by Tilo Neuendorf. Tilo Neuendorf and Ralf Haase prepared the figures. Ralf Haase, Nico Nitzsche, and Moritz Schumann commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplemementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Neuendorf, T., Haase, R., Schroeder, S. et al. Effects of high-intensity interval training on functional performance and maximal oxygen uptake in comparison with moderate intensity continuous training in cancer patients: a systematic review and meta-analysis. Support Care Cancer 31, 643 (2023). https://doi.org/10.1007/s00520-023-08103-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-08103-9