Abstract
Purpose
Little research has assessed cancer patients’ success criteria and priorities for symptom improvement to inform patient-centered care. Thus, we modified and tested a measure of these constructs for advanced lung cancer patients. We compared acceptable severity levels following symptom treatment across eight symptoms and identified patient subgroups based on symptom importance.
Methods
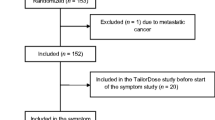
Advanced lung cancer patients (N=102) completed a one-time survey, including the modified Patient-Centered Outcomes Questionnaire (PCOQ), standard symptom measures, and other clinical characteristics.
Results
The modified PCOQ showed evidence of construct validity through associations with theoretically related constructs. Symptom severity and importance were moderately correlated. Levels of acceptable symptom severity were low and did not differ across the eight symptoms. Four patient subgroups were identified: (1) those who rated all symptoms as low in importance (n=12); (2) those who rated bronchial symptoms and sleep problems as low in importance and all other symptoms as moderately important (n=29); (3) those who rated nausea and emotional distress as low in importance and all other symptoms as moderately important (n=23); and (4) those who rated all symptoms as highly important (n=33). Subgroups were unrelated to clinical characteristics, except for functional status.
Conclusion
The modified PCOQ showed evidence of construct validity. Patients considered low symptom severity to be acceptable, irrespective of the symptom. Findings suggest that symptom severity and importance are related yet distinct aspects of the advanced lung cancer symptom experience. Patients have heterogeneous priorities for symptom improvement, which has implications for tailoring treatment.

Similar content being viewed by others
References
Reeve BB, Mitchell SA, Dueck AC, Basch E, Cella D, Reilly CM, Minasian LM, Denicoff AM, O’Mara AM, Fisch MJ (2014) Recommended patient-reported core set of symptoms to measure in adult cancer treatment trials. J Natl Cancer Inst 106(7):1–8. https://doi.org/10.1093/jnci/dju129
Rathert C, Wyrwich MD, Boren SA (2013) Patient-centered care and outcomes: a systematic review of the literature. Med Care Res Rev 70(4):351–379. https://doi.org/10.1177/1077558712465774
Robinson JH, Callister LC, Berry JA, Dearing KA (2008) Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract 20(12):600–607. https://doi.org/10.1111/j.1745-7599.2008.00360.x
Liang H, Tao L, Ford EW, Beydoun MA, Eid SM (2020) The patient-centered oncology care on health care utilization and cost: a systematic review and meta-analysis. Health Care Manag Rev 45(4):364–376. https://doi.org/10.1097/hmr.0000000000000226
Jayadevappa R, Chhatre S, Gallo JJ, Wittink M, Morales KH, Lee DI, Guzzo TJ, Vapiwala N, Wong YN, Newman DK, Van Arsdalen K, Malkowicz SB, Schwartz JS, Wein AJ (2019) Patient-centered preference assessment to improve satisfaction with care among patients with localized prostate cancer: a randomized controlled trial. J Clin Oncol 37(12):964–973. https://doi.org/10.1200/jco.18.01091
Barry MJ, Edgman-Levitan S (2012) Shared decision making—the pinnacle of patient-centered care. N Engl J Med 366(9):780–781. https://doi.org/10.1056/NEJMp1109283
te Boveldt N, Vernooij-Dassen M, Leppink I, Samwel H, Vissers K, Engels Y (2014) Patient empowerment in cancer pain management: an integrative literature review. Psychooncology 23(11):1203–1211. https://doi.org/10.1002/pon.3573
Jørgensen CR, Thomsen TG, Ross L, Dietz SM, Therkildsen S, Groenvold M, Rasmussen CL, Johnsen AT (2018) What facilitates “patient empowerment” in cancer patients during follow-up: a qualitative systematic review of the literature. Qual Health Res 28(2):292–304. https://doi.org/10.1177/1049732317721477
Wong ML, Cooper BA, Paul SM, Levine JD, Conley YP, Wright F, Hammer M, Miaskowski C (2017) Differences in symptom clusters identified using ratings of symptom occurrence vs. severity in lung cancer patients receiving chemotherapy. J Pain Symptom Manag 54(2):194–203. https://doi.org/10.1016/j.jpainsymman.2017.04.005
Brown JL, Edwards PS, Atchison JW, Lafayette-Lucey A, Wittmer VT, Robinson ME (2008) Defining patient-centered, multidimensional success criteria for treatment of chronic spine pain. Pain Med 9(7):851–862. https://doi.org/10.1111/j.1526-4637.2007.00357.x
Yi TI, Kim BK, Ha SA, Lim JY (2014) The relationships between determination of treatment success and emotional factors in patients with chronic musculoskeletal pain. Ann Rehabil Med 38(1):77–83. https://doi.org/10.5535/arm.2014.38.1.77
Nisenzon AN, Robinson ME, Bowers D, Banou E, Malaty I, Okun MS (2011) Measurement of patient-centered outcomes in Parkinson’s disease: what do patients really want from their treatment? Parkinsonism Relat Disord 17(2):89–94. https://doi.org/10.1016/j.parkreldis.2010.09.005
O'Brien EM, Staud RM, Hassinger AD, McCulloch RC, Craggs JG, Atchison JW, Price DD, Robinson ME (2010) Patient-centered perspective on treatment outcomes in chronic pain. Pain Med 11(1):6–15. https://doi.org/10.1111/j.1526-4637.2009.00685.x
Robinson ME, Brown JL, George SZ, Edwards PS, Atchison JW, Hirsh AT, Waxenberg LB, Wittmer V, Fillingim RB (2005) Multidimensional success criteria and expectations for treatment of chronic pain: the patient perspective. Pain Med 6(5):336–345. https://doi.org/10.1111/j.1526-4637.2005.00059.x
Rodrigue JR, Hanto DW, Curry MP (2011) Patients’ expectations and success criteria for liver transplantation. Liver Transpl 17(11):1309–1317. https://doi.org/10.1002/lt.22355
Stutts LA, Robinson ME, McCulloch RC, Banou E, Gremillion HA, Waxenberg LB, Staud R (2009) Patient centered outcome criteria for successful treatment of facial pain and fibromyalgia. J Orofac Pain 23(1):47–53
Zeppieri G, Lentz TA, Atchison JW, Indelicato PA, Moser MW, Vincent KR, George SZ (2012) Preliminary results of patient-defined success criteria for individuals with musculoskeletal pain in outpatient physical therapy settings. Arch Phys Med Rehabil 93(3):434–440. https://doi.org/10.1016/j.apmr.2011.10.007
Zeppieri G, Bialosky J, George SZ (2020) Importance of outcome domain for patients with musculoskeletal pain: characterizing subgroups and their response to treatment. Phys Ther 100(5):829–845. https://doi.org/10.1093/ptj/pzaa009
Tometich DB, Mosher CE, Hirsh AT, Rand KL, Johns SA, Matthias MS, Outcalt SD, Schneider BP, Mina L, Storniolo AMV (2018) Metastatic breast cancer patients’ expectations and priorities for symptom improvement. Support Care Cancer 26(11):3781–3788. https://doi.org/10.1007/s00520-018-4244-8
Iyer S, Roughley A, Rider A, Taylor-Stokes G (2014) The symptom burden of non-small cell lung cancer in the USA: a real-world cross-sectional study. Support Care Cancer 22(1):181–187. https://doi.org/10.1007/s00520-013-1959-4
Shin JA, Kosiba JD, Traeger L, Greer JA, Temel JS, Pirl WF (2014) Dyspnea and panic among patients with newly diagnosed non-small cell lung cancer. J Pain Symptom Manag 48(3):465–470. https://doi.org/10.1016/j.jpainsymman.2013.10.021
McGee SF, Zhang T, Jonker H, Laurie SA, Goss G, Nicholas G, Albaimani K, Wheatley-Price P (2018) The impact of baseline Edmonton Symptom Assessment Scale scores on treatment and survival in patients with advanced non-small-cell lung cancer. Clin Lung Cancer 19(1):e91–e99. https://doi.org/10.1016/j.cllc.2017.05.018
Kuon J, Vogt J, Mehnert A, Alt-Epping B, van Oorschot B, Sistermanns J, Ahlborn M, Ritterbusch U, Stevens S, Kahl C, Ruellan A, Matthias K, Kubin T, Stahlhut K, Heider A, Lordick F, Thomas M (2019) Symptoms and needs of patients with advanced lung cancer: early prevalence assessment. Oncol Res Treat 42(12):650–659. https://doi.org/10.1159/000502751
Cleeland CS, Zhao F, Chang VT, Sloan JA, O'Mara AM, Gilman PB, Weiss M, Mendoza TR, Lee JW, Fisch MJ (2013) The symptom burden of cancer: evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group Symptom Outcomes and Practice Patterns study. Cancer 119(24):4333–4340. https://doi.org/10.1002/cncr.28376
Batra A, Yang L, Boyne DJ, Harper A, Cheung WY, Cuthbert CA (2021) Associations between baseline symptom burden as assessed by patient-reported outcomes and overall survival of patients with metastatic cancer. Support Care Cancer 29(3):1423–1431. https://doi.org/10.1007/s00520-020-05623-6
Yount S, Beaumont J, Rosenbloom S, Cella D, Patel J, Hensing T, Jacobsen PB, Syrjala K, Abernethy AP (2012) A brief symptom index for advanced lung cancer. Clin Lung Cancer 13(1):14–23. https://doi.org/10.1016/j.cllc.2011.03.033
Dodd M, Janson S, Facione N, Faucett J, Froelicher ES, Humphreys J, Lee K, Miaskowski C, Puntillo K, Rankin S, Taylor D (2001) Advancing the science of symptom management. J Adv Nurs 33(5):668–676. https://doi.org/10.1046/j.1365-2648.2001.01697.x
Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC (2002) Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care 40(9):771–781. https://doi.org/10.1097/01.MLR.0000024610.33213.C8
Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S (2010) The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 63(11):1179–1194. https://doi.org/10.1016/j.jclinepi.2010.04.011
Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E, Sobel K, Coyle N, Kemeny N, Norton L (1994) The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer 30(9):1326–1336
Kroenke K, Theobald D, Norton K, Sanders R, Schlundt S, McCalley S, Harvey P, Iseminger K, Morrison G, Carpenter JS (2009) The Indiana Cancer Pain and Depression (INCPAD) trial: design of a telecare management intervention for cancer-related symptoms and baseline characteristics of study participants. Gen Hosp Psychiatry 31(3):240–253. https://doi.org/10.1016/j.genhosppsych.2009.01.007
Bauer J, Capra S, Ferguson M (2002) Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr 56(8):779–785. https://doi.org/10.1038/sj.ejcn.1601412
Cohen SR, Mount BM, Bruera E, Provost M, Rowe J, Tong K (1997) Validity of the McGill Quality of Life Questionnaire in the palliative care setting: a multi-centre Canadian study demonstrating the importance of the existential domain. Palliat Med 11(1):3–20. https://doi.org/10.1177/026921639701100102
Hagenaars JA, McCutcheon AL (2002) Applied latent class analysis. Cambridge University Press, New York
Muthén LK, Muthén BO (2017) MPlus user’s guide, 8th edn. Muthén & Muthén, Los Angeles
Clark SL, Muthén B (2009) Relating latent class analysis results to variables not included in the analysis. StatModel. https://www.statmodel.com/download/relatinglca.pdf. Accessed 29 June 2020
Celeux G, Soromenho G (1996) An entropy criterion for assessing the number of clusters in a mixture model. J Classif 13(2):195–212. https://doi.org/10.1007/BF01246098
Nylund KL, Asparouhov T, Muthén BO (2007) Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Model 14(4):535–569. https://doi.org/10.1080/10705510701575396
Vermunt JK (2010) Latent class modeling with covariates: two improved three-step approaches. Polit Anal 18(4):450–469. https://doi.org/10.1093/pan/mpq025
Butt Z, Rosenbloom SK, Abernethy AP, Beaumont JL, Paul D, Hampton D, Jacobsen PB, Syrjala KL, Von Roenn JH, Cella D (2008) Fatigue is the most important symptom for advanced cancer patients who have had chemotherapy. J Natl Compr Cancer Netw 6(5):448–455. https://doi.org/10.6004/jnccn.2008.0036
Henoch I, Ploner A, Tishelman C (2009) Increasing stringency in symptom cluster research: a methodological exploration of symptom clusters in patients with inoperable lung cancer. Oncol Nurs Forum 36(6):E282–E292. https://doi.org/10.1188/09.ONF.E283-E292
Choi S, Ryu E (2018) Effects of symptom clusters and depression on the quality of life in patients with advanced lung cancer. Eur J Cancer Care 27(1):e12508. https://doi.org/10.1111/ecc.12508
Kwekkeboom KL, Cherwin CH, Lee JW, Wanta B (2010) Mind-body treatments for the pain-fatigue-sleep disturbance symptom cluster in persons with cancer. J Pain Symptom Manag 39(1):126–138. https://doi.org/10.1016/j.jpainsymman.2009.05.022
Escalante CP, Manzullo EF (2009) Cancer-related fatigue: the approach and treatment. J Gen Intern Med 24(2):412–416. https://doi.org/10.1007/s11606-009-1056-z
Sturdza A, Millar B-A, Bana N, Laperriere N, Pond G, Wong RKS, Bezjak A (2008) The use and toxicity of steroids in the management of patients with brain metastases. Support Care Cancer 16(9):1041–1048. https://doi.org/10.1007/s00520-007-0395-8
Acknowledgements
The authors thank Gabriella Sblendorio for her assistance with this study.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable
Funding
This work was supported by the Walther Cancer Foundation (0172.01: Mosher) and the National Cancer Institute (T32CA117865: Krueger). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Walther Cancer Foundation or National Cancer Institute.
Author information
Authors and Affiliations
Contributions
Ellen Krueger, Ekin Secinti, and Catherine E. Mosher contributed to the study conception and design. Data collection was performed by Ellen Krueger and Ekin Secinti, and data analyses were performed by Ellen Krueger, Ekin Secinti, and Wei Wu. Nasser Hanna, Gregory Durm, Lawrence Einhorn, and Shadia Jalal assisted with data acquisition and interpretation. The first draft of the manuscript was written by Ellen Krueger and Catherine E. Mosher, and all authors reviewed, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Indiana University Institutional Review Board.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Participants provided informed consent regarding publishing their data.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Krueger, E., Secinti, E., Wu, W. et al. Measurement of patients’ acceptable symptom levels and priorities for symptom improvement in advanced lung cancer. Support Care Cancer 29, 5895–5904 (2021). https://doi.org/10.1007/s00520-021-06159-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06159-z