Abstract
Purpose
The aim was to prospectively map symptom clusters in patients with stage I–IIIa breast cancer during standard chemotherapy treatment in a randomised study.
Methods
Participants completed the Memorial Symptom Assessment Scale (MSAS) at baseline, day 12 after the first and third cycle of FEC 75 or FEC 100, and day 12 after the last cycle of Taxotere. Cut-off values for symptom scores, a mean value based on each individual reporting a symptom including occurrence, frequency, severity and distress for inclusion in analysis, were determined.
Results
The symptom burden cluster analysis was conducted in two steps and included symptoms with high frequency and high levels of distress. The factor analysis revealed three symptom clusters; physical, gastro (phys/gastro) and emotional, with core symptoms that remained stable over time. The most prevalent symptoms for the total sample during all cycles were as follows: lack of energy (range between 48 and 90%), feeling sad (48–79%), difficulty sleeping (54–78%), difficulty concentrating (53–74%), worrying (54–74%) and pain (29–67%).
Conclusion
In summary, we have prospectively established that symptom clusters remain stable over time with a basis of core symptoms. This knowledge will aid in the development of effective core symptom-focused interventions to minimise symptom burden for patients treated with chemotherapy for breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The emergence of pharmacogenetic studies in the field of clinical oncology has shed light onto the optimisation of anti-tumoral therapy, with the hope of individualising anti-cancer treatment. This approach should, if pharmacogenomic studies are properly applied, minimise the side effects of treatment while optimising treatment effects. Adjuvant chemotherapy is widely used to treat node-positive breast cancer, but a great inter- and intra-patient variability regarding side effects has been observed [20]. Most studies of cancer patients’ experience of side effects have reported side effects according to single symptoms. Patients treated for cancer do often, however, experience multiple concurrent symptoms that interact with each other and affect patient outcomes differently than the single symptoms [14]. The relevant literature reveals that most of the research on symptoms has been focused either on a single symptom, such as for example fatigue, or on their associated symptoms, such as anxiety and depression [25]. A symptom cluster is a condition where two or more symptoms that are related to each other occur simultaneously [6].
The research on groups or clusters of symptoms in cancer care has increased [22] one approach to clustering looks at the grouping of symptoms to identify symptoms which may occur together [21]. Symptoms within a cluster can have similar aetiology or a biological basis, and the treatment of one symptom in the clustering may have a positive influence on the occurrence of the other symptoms within the cluster as well [1].
Among women prior to surgery for breast cancer, fatigue, pain and sleep disturbance has been showed to form one cluster, and fatigue, pain, sleep disturbance and mild depression formed another [8]. In women with breast cancer during adjuvant chemotherapy treatment, the most distressing and burdensome clusters were made up of emotional, gastrointestinal and un-wellness symptoms (e.g., changes in skin, itching) [11]. Another study shows that a symptom cluster consisting of fatigue, depression and perceived cognitive impairment was found before, during and after completion of chemotherapy [26]; the studies also found low levels of quality of life in those patients with high symptom prevalence.
As symptom burden and distress is considered to be an important aspect of the symptom experience, symptom clusters should be considered when individualising breast cancer treatment [10]. Clusters may change with time as individuals undergo anti-tumour therapies [16]. There is, however, a paucity of prospective studies on symptom clusters in tailored chemotherapy for breast cancer. Thus, it is important to study possible symptom clusters during chemotherapy treatment, in order to better understand the full experience of side-effects at different points in time. Knowledge about symptom clustering can help healthcare professionals to be aware that other symptoms often occur alongside an already identified symptom. The aim of the present study was to describe symptom clusters at four points in time during chemotherapy treatment in patients with stage I–IIIa breast cancer.
Patients and methods
Patients
Newly diagnosed patients with histologically-confirmed stage I to IIIa breast cancer, participating in a randomised trial, the “Tailor Dose Trial” were included. The trial aimed to evaluate the usefulness of haemoglobin adduct to better tailor given doses of cyclophosphamide. The women were randomised to be treated with FEC (5-fluorouracil epirubicin cyclophosphamide) 75 (F600E75C600) at 3-week intervals × six, or to FEC 100 (F500E100C500) at 3-week intervals × three, followed by docetaxel × three times. Included patients should be able to give informed consent, read and speak Swedish, and to understand the purpose of the study. The exclusion criteria were known history of psychiatric disorder, non-Swedish-speaking, or a history of other cancer diagnoses within the previous 5 years.
Procedure
Data were collected by a study nurse at two clinics at the Department of Oncology, Karolinska University Hospital, Stockholm. The women were consecutively approached after surgery, before being randomised in the Tailored Dose Trial, the symptom study commenced after 20 patients had been included in the Tailored Dose Trial. The consort diagram is displayed in Fig. 1.
Consenting women were asked to complete the baseline questionnaires after written informed consent and thereafter at three points of assessment: day 12 after the first and third cycle of FEC75 or FEC 100, and day 12 after the last cycle of docetaxel, corresponding to 1, 3 and 6 months after inclusion in the study.
The instrument
Memorial symptom assessment scale
The MSAS scale was used to assess symptoms [24]. It includes 32 common cancer-related symptoms, assessed from the patient’s experiences during the past week. Twenty-four of the symptoms are evaluated with respect to frequency, severity, and distress. Eight symptoms are evaluated in terms of severity and distress. Each symptom is recorded as being either present or absent, and, if present, rated using a four-point scale (1–4) for frequency and severity, and a five-point scale (0–4) for distress during the previous 7 days. Higher scores indicate greater frequency, more severity, and higher distress. If a symptom is present, the symptom score is an average of the total of all scores within the rating scales. The scoring of the MSAS yields two subscale scores, including a physical symptom subscale score (PHYS-MSAS) and a psychological symptom subscale score (PSYCH-MSAS). A global distress index (GDI-MSAS) is also incorporated. The PHYS-MSAS includes 12 symptoms: lack of appetite, lack of energy, pain, feeling drowsy, constipation, dry mouth, nausea/vomiting, change in food taste, weight loss, feeling bloated, and dizziness. The PSYCH-MSAS includes six symptoms: worrying, feeling sad, feeling nervous, difficulty sleeping, feeling irritable, and difficulty concentrating. The GDI-MSAS includes four psychological symptoms (feeling sad, worrying, feeling irritable, and feeling nervous) and the distress associated with six physical symptoms (lack of appetite, lack of energy, pain, feeling drowsy, constipation, and dry mouth).
MSAS was regarded as the most encompassing subjective general symptom assessment instrument [7]. It has been used in clinical trials to assess the symptoms. The Swedish translation has shown sufficient validity and reliability [5].
Statistical analyses
Descriptive statistics were used to summarise socio-demographic and treatment data. The symptom score ranged between 0 and 4. The symptom score (symptom burden) was calculated as the average of the scores for the three dimensions (frequency, severity and distress), according to the MSAS manual [24], and was used to identify the appropriate cut-off level for inclusion. If data were missing for one of the three dimensions, the average was calculated based on the remaining two dimensions. If data were missing for two or three dimensions, the symptom burden was not calculated, and thus considered as missing. MSAS data were reported and analysed from 102 women. A cut-off point of 0.5 was chosen, based on results from a previous study, where associations were found between the presence of symptom clusters among patients and survival duration, independent of other prognostic factors [31]. This eliminated nine symptoms from further analysis; itching, weight loss, skin changes, swelling, swallowing difficulties, cough, numbness, urination problems and vomiting, because not reaching the cut-off point 0.5. The low “mean score” was based on low occurrence in combination with low levels of distress and severity. The symptom burden cluster analysis was conducted by means of factor analysis, including symptoms with high burden (symptom score above 0.5).
The factor analysis, employing principal component analysis as the extraction method and the varimax rotation method with Kaiser normalisation was conducted with the total symptom burden of the 23 remaining symptoms with a score above 0.5. The principal component analysis identified symptoms, those scoring highest burden, and the highest factor loading score that predicted the assignment to a “component” (cluster). The results were considered significant at the 5% level (p < 0.05). All calculations were carried out using IBM SPSS for Windows v. 22.
Results
From a consecutive sample of 243 eligible women, newly diagnosed with breast cancer, a total of 153 women agreed to participate in the Tailor Dose Trial. Of those, 124 patients (82%) accepted participation in the symptom assessment study (Fig. 1). Of the 124 participants, 67 were randomised to FEC 100 and 57 to FEC 75. The demographic and clinical characteristics of the participants are presented in Table 1.
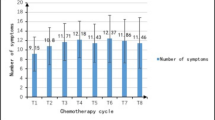
A majority of the women experienced several, but not all, of the symptoms assessed that contributed to the mean value of symptom scores. The most common mean symptom score value for the variables in this sample ranged between 0.5 and 1.5.
Symptom occurrence and distress
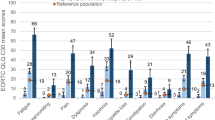
Patients who experienced symptoms reported the following problems in descending order during all cycles; “lack of energy” (range over the different treatment cycles 48–90%), “feeling sad” (48–79%), “difficulty sleeping” (54–78%), “difficulty concentrating” (53–74%), “worrying” (54–74%) and “pain” (29–67%). These were the most common symptoms among the women.
At baseline, “worrying” was the most common symptom (70%), and at all other cycles (one, three and sixth) it was “lack of energy” (76, 84, 85%). The most occurring symptoms, however, were not the most distressing. Symptoms that were less common at baseline, such as “mouth sores”(37%), “I don’t look like myself” (29%), “sexual interest” (28%), “sweats” (27%) were found to be the most distressing. After the last chemotherapy cycle problems with “taste change” (51%), “sexual interest” (44%), “sweats” (41%) were the most distressing symptoms.
Symptom clusters
Cluster 1 at baseline, the “Emotional cluster”, was characterised by the symptoms “worrying” (0.80), “difficulty concentrating”(0.78) and “feeling sad”(0.73). Cluster 2, the “Gastro cluster”, was comprised by symptoms of problems with “taste change” (0.90) “constipation” (0.65), and “diarrhoea” (0.44). Cluster 3 included “breathlessness”(0.63), “dizziness”(0.62), “dry mouth”(0.60) and “nausea”(0.55), defined by five symptoms of a physical nature and was labelled the “Physical cluster”.
At the first cycle of treatment the clusters were quite stable and similar to baseline. In the “Emotional cluster”, “feeling sad”(0.78) was the most predominant symptom. The most predominant symptom in the “Gastro cluster” was “lack of appetite” (0.76). The “Physical cluster” at this assessment point included “hair loss” (0.68) as the most predominant symptom.
The order of symptoms in the clusters changed at cycle 3. A mix of symptoms, including “lack of appetite” (0.75), “breathlessness” (0.64),“feeling nervous” (0.62),“lack of energy” (0.48), “feeling irritable” (0.48), “dizziness” (0.47), and now formed a “Physical cluster”. The most predominant symptom at this time point was “lack of appetite”(0.75). The “Emotional cluster” was dominated by “worrying” (0.67) and “feeling sad” (0.60), and the “Gastro cluster” by “mouth sores” (0.75) and “dry mouth” (0.67).
At cycle 6, the “Emotional cluster” was dominated by “feeling nervous” (0.80) followed by “worrying” (0.77), and “feeling sad” (0.73). The “Gastro cluster” comprised similar symptoms to those reported in the first cycle. The “Physical cluster” was dominated by problems with “sexual relations” (0.71) and also included “sweats” (0.53) and “difficulty sleeping” (0.56).
“Pain” and problems with “sexual relations” changed clusters over time by moving from the “Physical” to the “Emotional” cluster. Nine symptoms were excluded due to low symptom score (mean symptom score < 0.5). Low mean score is a result of low symptom occurrence in addition to a low distress score. The excluded symptoms are as follows: itching, weight loss, changes in skin, difficulty swallowing, swelling of arms or legs, cough, numbness/tingling in hands/feet, problems with urination and vomiting.
Discussion
Multiple symptoms in cancer patients present a complicated pattern of relationships. It is of vital importance to understand how symptoms interrelate in order to be able to implement effective interventions targeted at the related symptoms for maximal effect to minimise symptom burden for patients during treatment. Alleviating one single symptom seldom makes much clinical difference for the patient [13].
The clusters identified comprised a core of symptoms. Clusters are, however, dynamic constructs reflecting complex relationships and need a degree of flexibility and inclusiveness in the definition and construction, which has previously been identified [14]. It has been proposed that clusters with similar core symptoms across times and populations should be considered as consistent [14]. Interestingly the most distressing symptoms were not the most commonly occurring ones.
At baseline
The Physical cluster comprised, at baseline, symptoms of breathlessness, dizziness, nausea and dry mouth the core symptoms for this cluster remained similar in nature throughout the treatment cycles. In the “Emotional cluster ” patients in this study found being worried, sad and lack of appetite as most distressing. Similar symptom clusters of psychological character have previously been identified [4, 15, 17]. This cluster commonly include a variety of affective items depending on the scale used to elicit the symptom reports, and usually includes feelings of distress, sadness, lack of appetite, sleeping difficulties, irritability, anxiety, and depression [4, 15, 17]. Interestingly, these symptoms seem to cluster together in several cancer populations, both in the early and advanced setting and are as such of importance to target in clinical practice. Sadness and worry during cancer treatment together with physical changes, including hair loss after chemotherapy, are thought to cause shock and depression. Our study results are supported by the findings of others [32] showing that alopecia is connected to negative moods.
At cycle 1
Hair loss was the most predominant symptom in the Physical cluster at the first cycle and perceived as quite distressing. Alopecia has consistently been ranked in the literature as one of the most distressing symptoms of chemotherapy with evidence that some women refuse chemotherapy because of the risk of losing their hair [29]. This can give health care professionals direction of the importance of preparing patients for the physical impact of treatment, as well as follow-up assessment of the impact and coping ability of the individual patient as the hair loss occurs after the first cycle of treatment.
Similar to this study, Gastro clusters have previously been identified and commonly includes the items of nausea and vomiting [15, 18, 19] and lack of appetite or taste changes [15]. In the present study, vomiting was not present, which may be a result of the development and implementation of effective anti-emetic treatment. The connection between lack of appetite and energy, and dry mouth has also been observed in clustering previously [30] it was hypothesised that the connection between fatigue and dry mouth may indicate that fatigue is mediated by a dysfunction of the parasympathetic nervous system like for dry mouth.
At cycle 3
At the third treatment cycle there was an evident change in the connections between symptoms, lack of appetite was now more influenced by psychological issues such as worry and feeling sad. The gastrointestinal-fatigue and pain symptom clusters have been shown to affect functional status and overall aspects of quality of life in breast cancer patients [13].
At cycle 6
It is also important to identify unstable patterns in order to understand the dynamic nature of symptoms over time. Problems with sexual interest or activity and pain moved from the Physical cluster to the Emotional cluster as treatment progressed, indicating a closer connection with psychological symptoms. Problems with sexual interest had a lower prevalence than pain but higher distress values. One explanation for the higher distress value in connection to lack of sexual interest might be that the women worry that the lack of sexual interest will remain after treatment is completed. Sexual dysfunction of women with breast cancer undergoing chemotherapy has been shown to increase over time and even after treatment completion with the change of body image, and it may also be due to the systemic effects of chemotherapy or of abrupt, premature ovarian failure in younger women have probably a greater negative impact on sexuality than the effects of the local treatment for BC [23, 27].
Despite the differences in dosage of chemotherapy in FEC 75 versus FEC 100, we were not able to find any significant differences in symptom cluster burden between the two groups, which is not very surprising as the level of toxicity between the two treatments are quite similar (data not shown).
The present study confirms the existing knowledge on the clustering of symptoms. The identification of connections between symptoms over time may assist health care professionals in identifying symptoms and to set priorities in symptom management especially if time is restricted in the clinical setting. When identifying symptoms patients need to be assessed for related symptoms since these are likely to be present. In order to improve symptom management knowledge about how the treatment of one symptom positively may impact other symptoms is necessary for health care professionals.
Methodological considerations
Symptom clusters may vary according to the assessment of symptoms, the prevalence of symptoms within the sample, sample size, and sample composition [2]. The interpretation of results should be made cautiously because statistically derived clusters may be affected by several methodological considerations and clinical relevance can be difficult to determine [3]. In order to convincingly demonstrate the presence of a symptom burden cluster, the symptom cluster was considered present if patients reported at least 50% of symptoms within the symptom cluster. The symptom assessments were timed to match important landmarks of the treatment process. The choice was also made to use the MSAS scale for assessment of symptoms in order to capture prevalence, severity and distress. Interestingly, our data concur with previous studies in cancer populations in that the most distressing symptoms were not the most prevalent ones [9, 28]. This finding indicates that core symptoms within clusters should be identified because those seem to contribute most to the total symptom burden and causes of distress for patients [12].
Conclusion
In summary, by predefining score levels for symptoms for inclusion in analysis we have prospectively established that symptom clusters remain stable over time with defined core symptoms. This knowledge will aid in the development of effective interventions to minimise symptom burden for patients treated with chemotherapy for breast cancer. Future studies need to closely examine the relationships between multiple symptoms, specific interventions, and patient outcomes. Further research into symptoms and refinement of the definition of symptom clusters in the oncology population will aid in determining diagnostic criteria and the assessment, management, and prioritisation of care.
References
Aktas A, Walsh D, Rybicki L (2010) Review: symptom clusters: myth or reality? Palliat Med 24:373–385
Aktas A, Walsh D, Rybicki L (2012) Symptom clusters and prognosis in advanced cancer. Support Care Cancer 20:2837–2843
Barsevick AM (2007) The elusive concept of the symptom cluster. Oncol Nurs Forum 34:971–980
Bender CM, Ergyn FS, Rosenzweig MQ, Cohen SM, Sereika SM (2005) Symptom clusters in breast cancer across 3 phases of the disease. Cancer Nurs 28:219–225
Browall M, Sarenmalm EK, Nasic S, Wengström Y, Gaston-Johansson F (2013) Validity and reliability of the Swedish version of the memorial symptom assessment scale (MSAS): an instrument for the evaluation of symptom prevalence, characteristics, and distress. J Pain Symptom Manag 46:131–141. doi:10.1016/j.jpainsymman.2012.07.023
Charalambous A, Giannakopoulou M, Bozas E, Marcou Y, Kitsios P, Paikousis L (2016) Guided imagery and progressive muscle relaxation as a cluster of symptoms management intervention in patients receiving chemotherapy: a randomized control trial. PLoS One 11:e0156911. doi:10.1371/journal.pone.0156911
Davis MP, Kirkova J (2008) Lifting symptom burden—how far off the ground are we? Support Care Cancer 16:757–761
Denieffe S, Cowman S, Gooney M (2013) Symptoms, clusters and quality of life prior to surgery for breast cancer. J Clin Nurs 23:2491–2502
Deshields TL, Potter P, Olsen S, Liu J (2014) The persistence of symptom burden: symptom experience and quality of life of cancer patients across one year. Support Care Cancer 22:1089–1096
Kenne Sarenmalm E, Öhlén J, Jonsson T, Gaston-Johansson F (2007) Coping with breast cancer: predictors of distressing symptoms and health-related quality of life. J Pain Symptom Manag 34:24–34
Kenne Sarenmalm E, Browall M, Gaston-Johansson F (2014a) Symptom burden clusters: a challenge for target symptom management. A longitudinal study examining symptom burden clusters in breast cancer. J Pain Symptom Manag 47:731–741
Kenne Sarenmalm E, Browall M, Gaston-Johansson F (2014b) Symptom burden clusters: a challenge for targeted symptom management. A longitudinal study examining symptom burden clusters in breast cancer. J Pain Symptom Manag 47:731–741
Kim MY (2009) Transition of symptoms and quality of life in cancer patients on chemotherapy. J Korean Acad Nurs 39:433–445
Kim HJ, Abraham IL (2008) Statistical approaches to modeling symptom clusters in cancer patients. Cancer Nurs 31:E1–10
Kim HJ, Barsevick AM, Tulman L, McDermott PA (2008) Treatment-related symptom clusters in breast cancer: a secondary analysis. J Pain Symptom Manag 36:468–479
Kirkova J, Walsh D, Aktas A, Davis MP (2010a) Cancer symptom clusters: old concept but new data. American Journal of Hospice & Palliative Care 27:282–288
Kirkova J, Walsh D, Rybicki L, Davis MP, Aktas A, Tao J et al (2010b) Symptom severity and distress in advanced cancer. Palliat Med 24:330–339
Kirkova J, Aktas A, Walsh D, Rybicki L, Davis MP (2010c) Consistency of symptom clusters in advanced cancer. American Journal of Hospice & Palliative Care 27:342–346
Kirkova J, Rybicki L, Walsh D, Aktas A, Davis MP, Karafa MT (2011) The relationship between symptom prevalence and severity and cancer primary site in 796 patients with advanced cancer. American Journal of Hospice & Palliative Care 28:350–355
Miaskowski C, Cooper BA, Paul SM, Dodd M, Lee K, Aouizerat BE et al (2006) Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum 33:E79–E89
Miaskowski C, Aouizerat BE, Dodd M, Cooper B (2007) Conceptual issues in symptom clusters research and their implications for quality-of-life assessment in patients with cancer. JNCI Monogr:39–46
Molassiotis A, Wengström Y, Kearney N (2010) Symptom cluster patterns during the first year after diagnosis with cancer. J Pain Symptom Manag 39:847–858
Ochsenkühn R, Hermelink K, Clayton AH, von Schönfeldt V, Gallwas J, Ditsch N, Kahlert S (2011) Menopausal status in breast cancer patients with past chemotherapy determines long-term hypoactive sexual desire disorder. Journal of Sexual Medicine 8:1486–1494
Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E et al (1994) The memorial symptom assessment scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer 30A:1326–1336
Roscoea JA, Kaufmana ME, Matteson-Rusbyb SE, Palesha OG, Ryana JL, Kohlia A et al (2007) Cancer- related fatigue and sleep disorders. Oncologist 12(Supplement 1):35–42
Sanford SD, Beaumont JL, Butt Z, Sweet JJ, Cella D, Wagner LI (2014) Prospective longitudinal evaluation of a symptom cluster in breast cancer. J Pain Symptom Manag 47:721–730
Schover LR, Rhodes MM, Baum G, Adams JH, Jenkins R, Lewis P, Jackson KE (2011) Sisters peer counseling in reproductive issues after treatment (SPIRIT): a peer counseling program to improve reproductive health among African American breast cancer survivors. Cancer 117:4983–4992
Tishelman C, Lovgren M, Broberger E, Hamberg K, Sprangers MA (2010) Are the most distressing concerns of patients with inoperable lung cancer adequately assessed? A mixed-methods analysis. J Clin Oncol 28:1942–1949
Wagland R, Richardson A, Armes J, Hankins M, Lennan E, Griffiths P (2015) Treatment-related problems experienced by cancer patients undergoing chemotherapy: a scoping review. Eur J Cancer Care (Engl) 24:605–617. doi:10.1111/ecc.12246
Walsh D, Rybicki L (2006) Symptom clustering in advanced cancer. Support Care Cancer 14:831–836
Wikman A, Johar A, Lagergren P (2014) Presence of symptom clusters in surgically treated patients with esophageal cancer: implications for survival. Cancer 120:286–293
Yeon JH, Jung JY, Choi JW, Kim BJ, Youn SW, Park KC, Huh CH (2011) 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol 25:211–214. doi:10.1111/j.1468-3083.2010.03758.x
Acknowledgements
The authors acknowledge all the women taking part in this study, and thank Aileen Ireland for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval for the study was obtained from the Regional Ethics Committee, reference numbers 2011/1976-31/2, and 2012/1256-32.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study was supported by a grant from the Swedish Breast Cancer Organisation (BRO).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Browall, M., Brandberg, Y., Nasic, S. et al. A prospective exploration of symptom burden clusters in women with breast cancer during chemotherapy treatment. Support Care Cancer 25, 1423–1429 (2017). https://doi.org/10.1007/s00520-016-3527-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3527-1