Abstract
Purpose
Neutropenic complications remain the major dose-limiting toxicities of cancer chemotherapy. The aim of this study was to develop and internally validate a comprehensive and easily measurable scoring system for prediction of severe or febrile neutropenia in the first chemotherapy cycle of patients with solid tumors or lymphoma.
Methods
This prospective cohort study included consecutive patients at a tertiary referral hospital. Many clinical and laboratory-independent variables were measured at baseline. A multivariable logistic regression analysis was applied after unadjusted analysis, and the multivariable model was transformed into a simplified risk score based on 6 bootstrapped regression coefficients. The simplified scoring system was internally validated using cross-validation. All statistical tests were two-sided.
Results
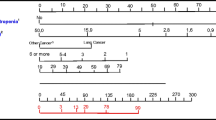
A total of 305 patients were enrolled and followed during 1732 chemotherapy cycles. Of these, 259 were eligible for analysis. The multivariable model revealed 6 predictive factors for severe or febrile neutropenia (scores in parentheses): high-risk regimen without colony-stimulating factor (4 points), intermediate-risk regimen without colony-stimulating factor (3 points), age > 65 years and elevated ferritin (3 points), body mass index < 23 kg/m2 and body surface area < 2 m2 (2 points), estimated glomerular filtration rate < 60 mL/min/1.73m2 (2 points), and elevated C-reactive protein (1 point). The receiver operating characteristic curve was 0.832 (95% confidence interval [Cl], 0.767–0.897) for the simplified model and 0.816 (95% Cl, 0.771–0.860) for the cross-validation.
Conclusions
We developed and internally validated a user-friendly prediction model to guide personalized decision-making using available clinical data and few cost-effective laboratory tests. External validation in other centers with different patients is required.


Similar content being viewed by others
References
Weycker D, Li X, Tzivelekis S, Atwood M, Garcia J, Li Y, Reiner M, Lyman GH (2017) Burden of chemotherapy-induced febrile neutropenia hospitalizations in US clinical practice, by use and patterns of prophylaxis with colony-stimulating factor. Support Care Cancer 25(2):439–447
Dinan MA, Hirsch BR, Lyman GH (2015) Management of chemotherapy-induced neutropenia: measuring quality, cost, and value. J Natl Compr Cancer Netw 13(1):e1–e7
Weycker D, Li X, Edelsberg J, Barron R, Kartashov A, Xu H, Lyman GH (2015) Risk and consequences of chemotherapy-induced febrile neutropenia in patients with metastatic solid tumors. J Oncol Pract 11(1):47–54
Culakova E, Thota R, Poniewierski MS, Kuderer NM, Wogu AF, Dale DC, Crawford J, Lyman GH (2014) Patterns of chemotherapy-associated toxicity and supportive care in US oncology practice: a nationwide prospective cohort study. Cancer Med 3(2):434–444
Kuderer NM, Lyman GH (2011) Personalized medicine and cancer supportive care: appropriate use of colony-stimulating factor support of chemotherapy. J Natl Cancer Inst 103(12):910–913
Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, Goldberg JM, Khatcheressian JL, Leighl NB, Perkins CL, Somlo G, Wade J, Wozniak A, Armitage J, American Society of Clinical Oncology (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33(28):3199–3212
Aapro MS, Bohlius J, Cameron D, Dal Lago L, Donnelly JP, Kearney N, Lyman G, Pettengell R, Tjan-Heijnen VC, Walewski J, Weber D, Zielinski C, European Organisation for Research and Treatment of Cancer (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47(1):8–32
Crawford J, Caserta C, Roila F, ESMO Guidelines Working Group (2010) Hematopoietic growth factors: ESMO clinical practice guidelines for the applications. Ann Oncol 21(suppl 5):v248–v251
Crawford J, Becker PS, Armitage JO, Blayney DW, Chavez J, Curtin P, Dinner S, Fynan T, Gojo I, Griffiths EA, Hough S, Kloth DD, Kuter DJ, Lyman GH, Mably M, Mukherjee S, Patel S, Perez LE, Poust A, Rampal R, Roy V, Rugo HS, Saad AA, Schwartzberg LS, Shayani S, Talbott M, Vadhan-Raj S, Vasu S, Wadleigh M, Westervelt P, Burns JL, Pluchino L (2017) Myeloid growth factors, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 15(12):1520–1541 doi:1540–1405
Aapro M, Ludwig H, Bokemeyer C, Gascón P, Boccadoro M, Denhaerynck K, Krendyukov A, Gorray M, MacDonald K, Abraham I (2016) Predictive modeling of the outcomes of chemotherapy-induced (febrile) neutropenia prophylaxis with biosimilar filgrastim (MONITOR-GCSF study). Ann Oncol 27(11):2039–2045
Pfeil AM, Vulsteke C, Paridaens R, Dieudonné A-S, Pettengell R, Hatse S, Neven P, Lambrechts D, Szucs TD, Schwenkglenks M, Wildiers H (2014) Multivariable regression analysis of febrile neutropenia occurrence in early breast cancer patients receiving chemotherapy assessing patient-related, chemotherapy-related and genetic risk factors. BMC Cancer 14(1):201
Chan A, Chen C, Chiang J, Tan SH, Ng R (2012) Incidence of febrile neutropenia among early-stage breast cancer patients receiving anthracycline-based chemotherapy. Support Care Cancer 20(7):1525–1532
Lyman GH, Kuderer NM, Crawford J, Wolff DA, Culakova E, Poniewierski MS, Dale DC (2011) Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer 117(9):1917–1927
Hosmer W, Malin J, Wong M (2011) Development and validation of a prediction model for the risk of developing febrile neutropenia in the first cycle of chemotherapy among elderly patients with breast, lung, colorectal, and prostate cancer. Support Care Cancer 19(3):333–341
Lopez-Pousa A, Rifà J, Casas de Tejerina A, Gonzalez-Larriba J, Iglesias C, Gasquet J, Carrato A, DELFOS Study Group (2010) Risk assessment model for first-cycle chemotherapy-induced neutropenia in patients with solid tumours. Eur J Cancer Care 19(5):648–655
Moreau M, Klastersky J, Schwarzbold A, Muanza F, Georgala A, Aoun M, Loizidou A, Barette M, Costantini S, Delmelle M, Dubreucq L, Vekemans M, Ferrant A, Bron D, Paesmans M (2009) A general chemotherapy myelotoxicity score to predict febrile neutropenia in hematological malignancies. Ann Oncol 20(3):513–519
Pettengell R, Bosly A, Szucs TD, Jackisch C, Leonard R, Paridaens R, Constenla M, Schwenkglenks M, Impact of Neutropenia in Chemotherapy-European Study Group (INC-EU) (2009) Multivariate analysis of febrile neutropenia occurrence in patients with non-Hodgkin lymphoma: data from the INC-EU prospective observational European neutropenia study. Br J Haematol 144(5):677–685
Lyman GH, Crawford J, Kuderer NM, Wolff D, Culakova E, Poniewierski MS, Dale DC (2008) Final risk prediction model for neutropenic complications in patients receiving cancer chemotherapy. Blood 112(11):1312–1312
Alexandre J, Rey E, Girre V, Grabar S, Tran A, Montheil V, Rabillon F, Dieras V, Jullien V, Hérait P, Pons G, Treluyer J, Goldwasser F (2007) Relationship between cytochrome 3A activity, inflammatory status and the risk of docetaxel-induced febrile neutropenia: a prospective study. Ann Oncol 18(1):168–172
Lyman GH, Abella E, Pettengell R (2014) Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit Rev Oncol Hematol 90(3):190–199
Alexandre J, Gross-Goupil M, Falissard B, Nguyen M-L, Gornet J-M, Misset J-L, Goldwasser F (2003) Evaluation of the nutritional and inflammatory status in cancer patients for the risk assessment of severe haematological toxicity following chemotherapy. Ann Oncol 14(1):36–41
Mehta HB, Mehta V, Girman CJ, Adhikari D, Johnson ML (2016) Regression coefficient-based scoring system should be used to assign weights to the risk index. J Clin Epidemiol 79:22–28
Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 162 (1):W1–73
Vandenbroucke JP, Von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M, Initiative STROBE (2007) Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med 4(10):1628–1654 (e1297)
Hosmer DW, Lemeshow S (2000) Assessing the fit of the model. In: Applied logistic regression, 2nd edn. Wiley, New York, pp 143–202
Hershman D, Hurley D, Wong M, Morrison VA, Malin JL (2009) Impact of primary prophylaxis on febrile neutropenia within community practices in the US. J Med Econ 12(3):203–210
Lyman GH, Delgado DJ (2003) Risk and timing of hospitalization for febrile neutropenia in patients receiving CHOP, CHOP-R, or CNOP chemotherapy for intermediate-grade non-Hodgkin lymphoma. Cancer 98(11):2402–2409
Scott S, Chrischilles E, Link B, Delgado D, Fridman M, Stolshek B (2003) Days of prophylactic filgrastim use to reduce febrile neutropenia in patients with non-Hodgkin's lymphoma treated with chemotherapy. J Manag Care Pharm 9(2 Supp A):15–21
Lyman GH, Sparreboom A (2013) Chemotherapy dosing in overweight and obese patients with cancer. Nat Rev Clin Oncol 10(8):451–459
Griggs JJ, Mangu PB, Anderson H, Balaban EP, Dignam JJ, Hryniuk WM, Morrison VA, Pini TM, Runowicz CD, Rosner GL, Shayne M, Sparreboom A, Sucheston LE, Lyman GH, American Society of Clinical Oncology (2012) Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 30(13):1553–1561
Barpe DR, Rosa DD, Froehlich PE (2010) Pharmacokinetic evaluation of doxorubicin plasma levels in normal and overweight patients with breast cancer and simulation of dose adjustment by different indexes of body mass. Eur J Pharm Sci 41(3):458–463
Li X, Luthra R, Morrow PK, Fisher MD, Reiner M, Barron RL, Langeberg WJ (2016) Comorbidities among patients with cancer who do and do not develop febrile neutropenia during the first chemotherapy cycle. J Oncol Pharm Pract 22(5):679–689
Chao C, Page J, Yang S-J, Rodriguez R, Huynh J, Chia V (2014) History of chronic comorbidity and risk of chemotherapy-induced febrile neutropenia in cancer patients not receiving G-CSF prophylaxis. Ann Oncol 25(9):1821–1829
Hurria A, Hurria A, Brogan K, Panageas KS, Pearce C, Norton L, Jakubowski A, Howard J, Hudis C (2005) Effect of creatinine clearance on patterns of toxicity in older patients receiving adjuvant chemotherapy for breast cancer. Drugs Aging 22(9):785–791
Nolin T, Naud J, Leblond F, Pichette V (2008) Emerging evidence of the impact of kidney disease on drug metabolism and transport. Clin Pharmacol Ther 83(6):898–903
Olyaei AJ, Foster TA, Lerma E (2015) Drug dosing in chronic kidney disease. In: Hornblower SRFS, Turner PNN, Goldsmith CND et al (eds) Oxford textbook of clinical nephrology, 4th edn. Oxford University Press, USA, pp 2911–2918
Hong J, Woo HS, Ahn HK, Sym SJ, Park J, Cho EK, Shin DB, Lee JH (2016) Pre-treatment blood inflammatory markers as predictors of systemic infection during induction chemotherapy: results of an exploratory study in patients with acute myeloid leukemia. Support Care Cancer 24(1):187–194
Kanda J, Mizumoto C, Ichinohe T, Kawabata H, Saito T, Yamashita K, Kondo T, Takakura S, Ichiyama S, Uchiyama T, Ishikawa T (2011) Pretransplant serum ferritin and C-reactive protein as predictive factors for early bacterial infection after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 46(2):208–216
Sato M, Kako S, Oshima K, Sato K, Terasako K, Kimura S-I, Nakasone H, Okuda S, Yamazaki R, Higuchi T, Nishida J, Kanda Y (2010) Prediction of infectious events by high-sensitivity C-reactive protein level before undergoing chemotherapy for acute myeloid leukaemia. Scand J Infect Dis 42(2):97–101
Sørensen J, Klee M, Palshof T, Hansen H (1993) Performance status assessment in cancer patients. An inter-observer variability study. Br J Cancer 67(4):773–775
Acknowledgements
This study was supported by Shahid Beheshti University of Medical Sciences [grant number 6620] and Behnam Daheshpour Charity Organization. The funders of this study were not involved in performing the study (e.g., data collection, analysis, and interpretation) as well as preparation of this report. We would like to thank Dr. A. Rezazadeh Mafi for critically reading the manuscript and Drs. H.R. Mirzaei, A. Rakhsha, A. Mousavizadeh, M. Malekzadeh, A.S. Yousefi Kashi, M. Houshyari, P. Hajian, and S. Azghandi for providing the patient data. We also thank the nursing staff of the oncology ward, especially Mrs. P. Peyghambarlou, for the administrative assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1
(DOCX 87.5 kb)
Rights and permissions
About this article
Cite this article
Razzaghdoust, A., Mofid, B. & Moghadam, M. Development of a simplified multivariable model to predict neutropenic complications in cancer patients undergoing chemotherapy. Support Care Cancer 26, 3691–3699 (2018). https://doi.org/10.1007/s00520-018-4224-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4224-z