Abstract
Management of food resources is considered fundamental for supporting insect pollinators, whose abundance shows a downward trend on the global scale. Here, the nectar and pollen production (per flower, per 1 m2 of tree crown), the composition of nectar carbohydrates and the levels of pollen proteins was evaluated in eight Tilia species (Malvaceae) growing in SE Poland. In the warm-summer continental climate, Tilia species can improve nectar and pollen resources mainly in June/July. Although the flowering period of each species is short (8.0–14.7 days), appropriate selection of species can extend the availability of food for more than a month. However, the considerable variations in the flowering onset (11.2–20.1 days) may cause significant inter-seasonal shifts in food accessibility.
The nectar with its highly changeable sugar concentration (29.5–77.4%) was composed of sucrose, glucose, and fructose; protein content in pollen was 7.2–16.8%. The mean sugar yield was in the range of 0.95–19.1 g per 1 m2 (T. amuriensis – T. platyphyllos, respectively), whereas the mean pollen yield was 1.37–4.1 g per 1 m2 of tree crown (T. amuriensis – T. × euchlora, respectively). Significant year-to-year fluctuations of sugar and pollen yield in linden trees have to be taken into account in conservation schemes, and the introduction of other flowering plants with more stable sugar and pollen production should be considered in an area with a high density of linden trees. Linden flowers mainly support honey bees; nevertheless, wild pollinators (bumble bees, solitary bees, and dipterans) can also benefit from linden floral resources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trees constitute an ecologically and aesthetically important component of diverse ecosystems (Czaja et al. 2020). In urban ecosystems, they improve city climate, absorb stormwater, reduce air pollution and smog, store carbon, decrease the energy that reaches the ground, and enhance biocontrol (Nowak et al. 2018; reviewed in Donkersley 2019). There is also evidence that trees provide health benefits for local residents, e.g. support convalescence after stress and illness and reduce mental fatigue (Ulrich et al. 1991; Li and Sullivan 2016).
Recently, attention has been paid to the importance of trees in maintaining the diversity and abundance of insect pollinators (Bożek et al. 2023 and literature cited therein). Pollinator conservation contributes to global management efforts, as pollinator decline is reported all over the world (Ayers and Rehan 2021). The potential threats to pollinators are derivative of the loss and fragmentation of habitats, agricultural intensification and use of chemicals (pesticides, herbicides), intensification of urbanization, environmental pollution, climate change, diseases, and pests (Baldock 2020). However, the loss of floral resources and homogenization of diet are mentioned as critical factors responsible for the pollinator decline (Goulson et al. 2015). The availability of food resources demonstrates seasonal and annual variation and differs substantially between regions and landscapes (Timberlake et al. 2019; Baldock 2020). These differences are mainly derivative of variations in the abundance and diversity of flowering plants and the differences in flowering phenology (flowering time and duration) (Daniels et al. 2020; Bożek et al. 2023). A serious problem in many types of landscapes is ‘food gaps’ (i.e. periods with no available floral resources) (Vaudo et al. 2015). Recently, it has also been indicated that plant species with an optimal “nutritive value” are necessary for the proper development of pollinators (Filipiak et al. 2017; Filipiak 2019).
Various traits of floral reward may have an impact on attractiveness for pollinators, e.g. floral morphology, features of the nectar (amount, sugar concentration, volatile composition; e.g. Nicolson and Thornburg 2007; Masierowska et al. 2018; Tew et al. 2021). In general, plant species differ in the amount of produced resources and their nutritional value (Somme et al. 2015; Vaudo et al. 2015; Bożek 2021; Dmitruk et al. 2022). Nectar and pollen are the dominant food resources for pollinators (e.g. Nicolson 2011). Nectar is regarded as the main energy source. It is composed of three common carbohydrates (glucose, fructose, and sucrose) (Nicolson 2011). However, nectar characteristics are more complex and include the amount of secretion, the nectar sugar concentration, the proportion of sugars, and the content and composition of amino acids or secondary metabolites (Bernardello 2007). Nectar traits (mainly the nectar amount and the sugar concentration) can be seriously affected by environmental factors (e.g. water availability, air temperature and humidity, light); therefore, intra-species variations in nectar characteristics are reported [e.g. (Corbet et al. 1979; Farkas and Zajácz 2007; Donkersley 2019)].
Pollen is regarded as the prime nutrient resource that provides insects with proteins, lipids, macro- and microelements, vitamins, hormones, carotenoids, and flavonoids (Roulston and Cane 2000; Nicolson 2011; Di Pasquale et al. 2016). Individual flowers differ considerably in pollen productivity, which is related to androecium morphology (i.e. number of anthers per flower, anther size) (Szklanowska and Teper 1999; Masierowska et al. 2018; Dmitruk et al. 2022). Also, the pollen nutritional value varies substantially from species to species; for example, the crude protein content is in the range of 2–60% (Crailsheim et al. 1992; Roulston and Cane 2000). Both the amount of produced pollen and its chemical composition are important for the proper development of pollinators and brood rearing (e.g. (Di Pasquale et al. 2016).
Planting trees/shrubs is especially recommended for the improvement of food resources for pollinators as a highly cost-effective and long-term approach (e.g. Filipiak 2019; Donkersley 2019; Tew et al. 2021; Bożek et al. 2023). Trees are strongly recommended, as they are suggested to offer more critical food sources than herbaceous plants (Geslin et al. 2013). The superlative role of trees is related to the large number of developed flowers that can provide pollinators with abundant nectar and pollen (Baude et al. 2016; Daniels et al. 2020). Trees can also help to complement the pollinator food base and fill the seasonal ‘food gaps’ (Donkersley 2019). Several authors have also emphasized that woody species offer high-value nectar and pollen resources (Vaudo et al. 2015; Hall et al. 2017; Filipiak 2019). However, there are also reports that indicate that trees may provide inadequate or even toxic floral resources (Pawlikowski 2010; Koch and Stevenson 2017).
Activities towards the management of pollinator food resources require the creation of a list indicating attractive plant species for diverse groups of pollinators (Theodorou et al. 2020). Such a list should contain top nectar and pollen producers (Hall et al. 2017; Ayers and Rehan 2021; Tew et al. 2021). The list should also be supplemented with other information, e.g. the time and duration of blooming, groups of pollinators that use floral resources, plant status (native, exotic, naturalized, or invasive), and other values (decorative, ecological) (Dmitruk 2019; Ghosh et al. 2020; Dmitruk et al. 2022). The information is essential for policy makers and can help to optimize sustainable bee conservation (Phillips et al. 2020; Baldock 2020; Tew et al. 2021; Braman and Griffin 2022).
The genus Tilia includes ca. 60 species that are native to the temperate zone of the Northern Hemisphere. However, there are also species cultivated in southern Africa, e.g. T. americana, T. × euchlora, T. cordata, T. platyphyllos, and T. tomentosa (Glen 2002). Moreover, there is a great number of cultivars and hybrids of Tilia species (Seneta and Dolatowski 2012). Native and exotic Tilia species tend to be used across large geographical areas due to their aesthetic value (Ignatieva 2011; Grote et al. 2016). Furthermore, they are listed among the 10 top phytoremediants with a high capacity for the reduction of dust pollutants (in particular T. platyphyllos and T. cordata) and are recommended for use in urban areas (Popek et al. 2013). They support the mitigation of the ‘heat island effect’ and improve city climate (Nowak et al. 2018). The other advantageous traits of Tilia species are their tolerance to temperature changes as well as drought- and pest-resistance (Sadowiec and Gawroński 2013; Bayer et al. 2018; Kusiak et al. 2022). As very popular urban trees that produce a large number of flowers, lindens have a great potential impact on pollinators within diverse landscape types, including urban areas (Baude et al. 2016; Daniels et al. 2020). Therefore, there is a need to evaluate their usefulness as a food base.
The current study focused on the determination of (i) the time of flowering and (ii) production of nectar sugars and pollen in the flowers and per 1 m2 of tree crown of eight species from the genus Tilia. The profile of nectar carbohydrates and the quantity of pollen proteins were also evaluated to complete the floral reward characterization. We also attempted to assess the attractiveness of individual species for insect visitors (based on the composition and abundance of pollinator taxonomic groups).
Material and methods
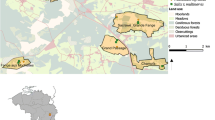
The observations were conducted in 2018–2019 and in 2021 in Lublin, SE Poland (51°08’–51°18’N and 22°27’–22°41’E; Fig. 1). Lublin—the capital of Lublin Voivodeship, is one of the largest cities in Poland with ca. 350,000 inhabitants. It ranks fifth in the general national classification of sustainable cities (Borys et al. 2021). The city is characterized by a significant share of green infrastructure coverage (ca. 40%), including parks, urban meadows, forests (deciduous and coniferous pine), tree avenues, greenways, and private green spaces). The region is located in a warm-summer continental climate zone (Dfb in Köppen classification; Chen and Chen 2013). The mean annual temperature is + 8.9 °C (48.0 °F) and the mean annual rainfall is 750 mm. The coldest month is January (mean air temperatures = 3.6 °C; 38.4°F) and July is the warmest month (mean = 18.6 °C; 65.5°F). Snow cover remains for 60–80 days per year. The vegetation period in the Lublin area lasts ca. 209 days (https://pl.climate-data.org). The annual concentration of PM2.5 of approx. 12.1–35.4 μg/m3 is higher than in many European cities (https://www.iqair.com).
The distribution of individual trees of linden species is shown in Fig. 1. We selected eight species from the genus Tilia L. (i) native to Polish flora (T. platyphyllos Scop., T. cordata Mill.) and (ii) exotic species: T. americana L. (native to Canada and the central and eastern United States), T. dasystyla Steven (occurring in areas from the western part of Russia to Iran), T. amurensis Rupr. (native to eastern Asia), T. tomentosa Moench ‘Petiolaris’ (occurring in southeastern Europe and southwestern Asia), T. japonica (Miq.) Simonk (native to eastern China and Japan), and Tilia × euchlora (= T. dasystyla × T. cordata; cultivated, not occurring in the wild). The number of individuals included in the observations was n = 4 (T. platyphyllos, T. americana, T. cordata), n = 3 (T. × euchlora, T. amurensis, T. tomentosa ‘Petiolaris’), and n = 2 (T. dasystyla, T. japonica).
According to the Cronquist classification system, Tilia spp. belong to the family Tiliaceae Juss. (linden family). In turn, in the APG IV classification, the genus belongs to the family Malvaceae Juss. (mallow family). The species from the genus Tilia are deciduous trees. Most of these species are native to the Northern Hemisphere (mainly Europe or North America). The flowering of linden trees propagated generatively begins at the age of about 25–30 years, but those originating from vegetative sprouts blossom at about 10–15 years (Radoglou et al. 2009; Seneta and Dolatowski 2012). Linden flowers are fragrant, yellow, and easily accessible for insect visitors (Anderson 1976). They offer nectar and pollen (Szklanowska and Teper 1999).
Flowering
We observed the phenology of flowering, i.e. the trees were monitored every 2–3 days from mid-May to the end of July, for 2–4 individuals depending on the species. We followed the procedure described in detail by Dmitruk et al. (2022). In brief, the date of the onset of flowering and the date of the end of flowering were recorded (onset—5% of flowers in bloom; end—ca. 80% of flowers wilted).
The average number of flowers per 1 m2 of tree crown was estimated based on the number of flowers in an inflorescence (randomly selected, n = 10 per species, per year) and the number of inflorescences in an area of 0.1 m2 (n = 6). An area of 0.1 m2 was determined using a ring with a diameter of 36.7 cm (Denisow 2011); then, the number of flowers was recalculated per 1 m2.
Nectar measurements
To establish the amount of secreted nectar, the branches/inflorescences on different trees (n = 2–4, depending on the species) were bagged with isolators (tulle type) to exclude pollinators. Nectar was removed from randomly selected flowers using glass pipettes with known mass (Jabłoński 2002). Nectar from each species was sampled twice per season in three replications. The flowers were picked at the same time of the day (in the morning hours 9.00–10.00 GMT + 2 h) and immediately transferred to the laboratory. Each replication consisted of nectar from 10 to 12 flowers. The nectar samples were weighed using an analytical balance and expressed in mg. Its sugar concentration was measured with a digital refractometer (in % w/w). Then, the nectar sugar amount was determined (in mg).
Nectar carbohydrate analysis
The technique of high-performance liquid chromatography (HPLC) was employed (Shimadzu system, USA) to identify and quantify the types of nectar carbohydrates. Mean values of carbohydrates were calculated and were shown as a percentage of total sugars (± SD). Next, the sugar ratios were calculated (S/G + F and G/F; where: S—sucrose G—glucose, F—fructose) (Bernardello 2007; Nicolson and Thornburg 2007).
Pollen measurements
The amount of produced pollen was established using the method described in detail by Denisow (2011). Buds (randomly selected) from different individuals (n = 2–4, depending on the species) were collected. Each year, the samples (n = 6; with 100 anthers per sample) were gathered per species per year. The anthers (well-developed but before dehiscence) were extracted from the buds and transferred into glass containers with known tare weight. Then, the glass containers with the samples were transferred into a dryer for 78 h (temperature ca. 31–34 °C). After the anthers opened, the pollen was washed out using 2–6 mL of ether and 3–6 mL of 70% ethanol. The procedure was repeated 4 times. The glass containers with pollen separated from anther tissues were placed in a dryer again (for one-two weeks). To establish the pollen dry weight, the samples were reweighed. The mass of produced pollen was established per one flower. To this end, the number of anthers was counted (n = 15 flowers).
The pollen quality was evaluated by means of assessment of the protein content according to the Kjeldahl method (Bradford 1976). First, the total nitrogen – N was measured in dry pollen samples (n = 4 per species). A factor of 6.25 was used to calculate crude protein content (Roulston and Cane 2000).
Insect visitors
In 2019 and 2021, insect visitors were observed to establish whether they feed on the floral reward provided by the Tilia species studied. Insect visitors were counted taking into account particular taxonomic groups: Apis mellifera, Bombus spp., solitary bees, and Diptera. These observations were made for three days in the full-bloom phase. The counts were made on branches accessible for observation. We counted the insect visitors in different periods of the day (9.00–10.00, 12.00–13.00, and 17.00–18.00 GMT + 2 h; n = 6 counts per day for circle areas with a diameter of 36.7 cm, i.e. 0.1 m2; n = 18 counts per season, per species; Denisow 2011). The census of observations was made for ca. 10 min. To compare the insect groups abundance, the data from diverse individuals of the same species and periods were summed. Insect monitoring was conducted in suitable weather conditions (no wind, no rain, air temperature 20–28 °C).
Statistical analyses
All data are presented as mean ± SE. For phenological data analyses, the recorded date of flowering phenology (start and end of flowering) was converted into a Julian date—day of the year (Jabłońska et al. 2015). We applied the non-parametric Kruskal–Wallis H test (for more than two independent samples with different sample sizes) for comparing the start and end dates of flowering and flowering period durations between species and between years.
The data on the flowering abundance, number of anthers, and the nectar and pollen production were verified for normality (the Shapiro–Wilk test) before the analyses were performed. These data were log transformed, as the normal distribution was not confirmed. Next, the two-way analysis of variance (ANOVA) was applied to test the differences (for the species and the year effects and for the interaction between these two factors) in the analyzed traits (abundance of flowering, number of anthers per flower, nectar and pollen data). Post-hoc means were compared with the use of Tukey's test (α = 0.05).
The results on the protein content in pollen were verified with the Lilliefors test, which indicated the lack of homogeneity of variance. Therefore, the non-parametric Kruskal–Wallis H test was used to check differences in the protein content in the pollen between the species.
Spearman’s correlation coefficients were established to describe the statistical dependence between different variables (sugar mass per flower vs. nectar mass per flower; sugar mass per flower vs. nectar sugar concentration; total sugar/pollen yield per 1 m2 vs. nectar traits vs. flowering abundance). For interpretation of the correlations, the following guide was applied: 0.0–0.19—very weak; 0.20–0.39 – weak; 0.40–0.59 – moderate; 0.60–0.79 – strong, and 0.80–1.0 – very strong. The data presented in the tables and graphs are untransformed means (± SD, standard deviation). These analyses were performed using Statistica software (v. 13.3, Statsoft Poland).
To assess the dominant flower visitor groups for Tilia species in our study sites, we used the non-parametric Kruskal–Wallis H test for independent variables. Pearson’s chi-square tests for independence and goodness of fit were used to examine the distributional properties of the insect visitor data. We tested whether the observed distributions of insect visitors for particular Tilia species fit the uniform distribution. These calculations and graphics were performed in the R environment (R ver 4.3.1).
Results
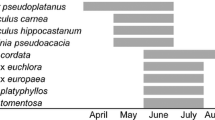
The flowering periods of the Tilia species varied (Fig. 2, Table S1- Supplementary materials).
Most Tilia species were at the flowering peak in June.
The onset of flowering differed between the species (H (7, N = 75) = 31.82, P < 0.001). Four species (T. platyphyllos, T. americana, T. dasystyla, T. cordata) were classified in the early flowering group. Also, there were differences in the flowering onset date between the years of the study (H (2, N = 75) = 37.59, P < 0.001). Acceleration of the flowering onset was documented in 2018, in which the flowering start was registered 20.1 days earlier than in 2021 and 11.2 days earlier in relation to 2019. The disparity in the flowering end ranged from 9.9 to 18.9 days between the years. No difference was revealed for the flowering duration (species: H (7, N = 75) = 13.74, P = 0.056; year H (2, N = 75) = 2.68, P = 0.269). On average, the duration of linden flowering was 11.8 days.
Significant differences in the number of flowers developed per 1 m2 were recorded between the Tilia species (F7,120 = 25.00, P < 0.001), between the study years (F2,120 = 36.37, P < 0.001) and for species x years interaction (F14,120 = 2.37, P = 0.006; Table S2). The number of flowers produced per Tilia species was on average 3.6 ± 2.9 thousand per m2 of tree crown. The largest number of flowers was recorded in T. cordata (7.3 ± 4.5 thousand). Less abundant flowering was noted for T. tomentosa ‘Petioralis’ and T. amurensis (1.3 ± 0.6 and 1.6 ± 0.9 thousand, respectively). The highest number of flowers was established in 2019, a ca. 30% lower abundance of flowers was noted in 2018, and the fewest flowers were recorded in 2021.
Nectar sugar and pollen production in flowers
The nectar traits varied across the Tilia species and the years of the study (for nectar mass secreted per flower—F7,120 = 53.02, P < 0.001, F2,120 = 13.42, P < 0.001, species x years F14,120 = 2.97, P < 0.001; for the nectar sugar concentration—F7,120 = 3.30, P = 0.003, F2,120 = 22.48, P < 0.001, species x years F14,120 = 4.43, P < 0.001; for nectar sugar per flower—F7,120 = 35.44, P < 0.001, F2,120 = 1.69, P < 0.001, species x years F14,120 = 1.30, P = 0.213; Table 1).
In terms of the nectar amount, the flowers of T. amurensis and T. japonica produced low amounts of nectar (1.74 ± 1.2 mg and 4.74 ± 1.8 mg per flower), In contrast, T. tomentosa “Petiolaris’, T. × euchlora, T. americana, T. platyphyllos, and T. dasystyla were classified as high nectar producers (they secreted 7.97 ± 3.5 mg—11. 47 ± 3.2 mg of nectar). The comparison of the sugar concentration in the nectar showed a range of 22.24%–77.38% between the different species. The highest amount of sugars was produced in the flowers of T. platyphyllos, T. × euchlora, T. tomentosa ‘Petiolaris’, and T. americana, while the lowest amount of sugars was recorded in T. amurensis. In every species, the lowest sugars mass was found in 2021. The sugar mass per flower was strongly correlated with the nectar mass secreted in the flowers (R = 0.8526, P < 0.05) and moderately correlated with the nectar concentration (R = 0.4354, P < 0.05).
The pollen mass in the Tilia flowers varied between the species (F7,120 = 21.25, P < 0.001; Table 1) and was positively correlated with the number of anthers per flower (R = 0.3687, P < 0.05; Table 2). The species (F7,216 = 61.8, P < 0.001), year (F2,216 = 48.6, P < 0.001) and species x years (F14,216 = 12.0, P < 0.001) effects where significant for the number of anthers developed per flower. The lowest number of anthers were found in T. japonica and T. cordata (23.8 ± 2.6 and 27.1 ± 3.3, respectively), whereas the highest number was recorded in T. americana (58.6 ± 5.2; Table S2). The amount of pollen per flower varied from 0.20 ± 0.08 mg to 1.34 ± 0.30 mg. On average, one Tilia flower produced 0.78 ± 0.46 mg of pollen. The highest amount of pollen was produced in the flowers of T. × euchlora and T. tomentosa ‘Petiolaris’ (1.23 ± 0.33 mg and 1.18 ± 0.30 mg per flower, respectively). A small amount of pollen was recorded in the flowers of T. cordata and T. japonica (0.37 ± 0.39 mg and 0.32 ± 0.27 mg per flower, respectively). We did not observe the year effect (F2,120 = 0.762, P = 0.4690) and any interaction between the species and the study years (F14,120 = 1.605, P = 0.087) for milligrams of produced pollen.
Total sugars and pollen yield
The total sugar yield per 1 m2 of tree crown differed across the Tilia species studied (F7,120 = 94.95, P < 0.0001; Fig. 3A). The Tilia species produced on average 8.5 ± 0.8 g of sugars per 1 m2 of tree crown. T. × euchlora and T. platyphyllos were the top-ranked producers (19.1 ± 10.4 g per 1 m2 and 14.7 ± 9.0 mg per 1 m2, respectively). The lowest amount of total sugars was calculated for T. amurensis (0.95 ± 0.7 g per 1 m2). The year effect for the yield of sugars was significant (F2,120 = 79.80, P < 0.001). The lowest total sugar yield was calculated for 2021 (2.9 ± 1.1 g per 1 m2), and it was ca. 3–fourfold lower than in the other study years. The species x years interaction was also significant (F14,120 = 3.43, P < 0.001). The sugar production per 1 m2 was strongly positively correlated with the mass of sugars secreted by the flowers (R = 0.8918 P < 0.05), and there was a moderate positive correlation with the number of flowers (R = 0.367, P < 0.05) (Table 2).
Total yield of nectar sugar (A) and pollen (B) in eight Tilia species in the years of the study (in SE Poland). Means marked by different letters differ significantly: A-C between species, X–Z between years, a-d species x years; Tukey’s HSD test performed for log transformed data P < 0.05. The presented values are untransformed; the whiskers show SD- standard deviation
The total amount of pollen produced per 1 m2 of Tilia crown differed between the species (F7,120 = 13.17, P < 0.001; Fig. 3B). On average, 1 m2 of Tilia tree crown produced 2.2 ± 1.5 g of pollen. T. × euchlora, yielding 4.1 ± 1.9 g of pollen per 1 m2 of crown, was the top-ranked producer. The other species yielded ca. 50–60% less pollen per 1 m2 of crown. The total yield of pollen differed between the study years (F2,120 = 70.56, P < 0.001; species x years interaction (F14,120 = 2.84, P < 0.001). The highest amount was noted in 2019 (3.3 ± 1.5 g per 1 m2). In the other years, a 30–55% decrease in the total pollen yield was recorded. The total pollen yield per 1 m2 was positively correlated with the number of developed flowers (R = 0.6117, P < 0.05) (Table 2).
Nectar quality
The HPLC analyses detected only hexoses (glucose and fructose) and sucrose in the nectar of the studied Tilia species (Table 3). The concentrations of glucose and sucrose in the nectar differed significantly between the species (glucose: F7,8 = 28.37, P < 0.001; sucrose: F7,8 = 11.67, P < 0.001), while the fructose content did not differ (F7,8 = 1.31, P = 0.355). The sucrose/hexose ratio was species dependent (F7,8 = 9.43, P = 0.002) and ranged from 0.36 ± 0.08 to 1.49 ± 0.76.
The protein content in the pollen was in the range of 7.2–16.8% (11.2 ± 5.4%). We found no significant differences in the content of proteins for individual species (chi-squared = 11.483, df = 7, P = 0.1189) (Fig. 4).
Insect visitors
We identified four insect groups visiting the flowers the Tilia species studied (Apis mellifera, Bombus species, solitary bees, and dipterans) (Fig. 5). The abundance of the insect visitors differed (H (3, N = 32) = 28.91, P < 0.001). The dominant visitors were Apis mellifera (314.1 ± 43.8), followed by Bombus spp. (81.3 ± 0.7).
Using the chi-square test, we found that the frequency of particular insect groups varied significantly between the Tilia species (χ2 = 40.002, df = 21, P = 0.0074). In both study years, the distribution of honey bees differed between the linden species (2019: χ2 = 76.819, df = 7, P = 0.0003; 2021: χ2 = 121.61, df = 7; Fig. 6); however, the preferences changed among the years, e.g. T. amurensis and T. dasystyla were visited the most willingly in 2019, whereas T. platyphylos was preferred in 2021. The Bombus species showed variability in the visit frequencies among the linden species only in 2019. The empirical frequencies were found to match those expected from the uniform model in the following cases: Diptera/2019 (P = 0.4711), Bombus/2021 (P = 0.171), solitary bees/2021 (P = 0.9731), and Diptera/2021 (P = 0.7006), which indicates that there was no preference for one Tilia species over another.
Bubble plot illustrating the distribution of the insect visitor groups among the Tilia species in Lublin, Poland, in (A) 2019 and (B) 2021. Pearson Chi-squared test: Apis mellifera 2019 and 2021 – P < 0.0001, Bombus 2019 – P < 0.0001, Bombus 2021 – P = 0.171; solitary bees 2019 – P = < 0.0001, solitary bees 2021 – P = 0.9731; Diptera 2019 – P < 0.0001, Diptera 2021 – P = 0.7006
Discussion
In the present study, the potential of eight Tilia species as a food resource for pollinators was estimated. We also tried to evaluate the possibility of using these species to improve the insect food base in Poland. Therefore, we characterized the flowering phenology, as it can illustrate the periods of availability of nectar and pollen resources and/or their deficit. We also assessed the amount of available sugar and pollen in flowers and estimated the total resources offered per unit area. Admittedly, our research has limitations, e.g. the study concerned a limited number of individuals (only 2–4 depending on the species) grown in one city. The observations of insect visitors covered only a part of the phenological season, we noted only the insect visitors visible in the observer’s sight and no detailed observations of co-flowering plants were carried out. In the case of the flowering dates, it should be emphasized that the presented data are short-term (they cover only three years).
Flowering periods
In Western and Central Europe, most Tilia species provide nectar and pollen in June and/or July (Szklanowska and Teper 1999; Farkas and Zajácz 2007; Jacquemart et al. 2018; Weryszko-Chmielewska et al. 2019; Czaja et al. 2020), which is also consistent with the flowering time established in our study region. In SE Poland, the availability of woody ornamental plants that can supplement food for insects is limited during June and/or July periods. For example, Robinia pseudoacacia L., Symphoricarpos albus Duhamel, Gleditsia triacanthos L., Lonicera spp., and Irga spp. can support pollinators in June. In turn, the number of woody species flowering in July is even narrower, and linden species are then the main flowering trees. Therefore, linden trees should be promoted more widely to improve the food base for insects in urban environments in Central, Eastern, and Western Europe.
Our short-time analysis has also shown that there may be 2–3-week (11.2–20.1 days) differences in the flowering onset of individual Tilia species between seasons, and flowering may even start as early as the end of May. This disparity is not surprising, since other authors have highlighted that Tilia species flowering is sensitive to weather conditions (e.g. Jabłoński and Kołtowski 1999; Szklanowska et al. 1999; Weryszko-Chmielewska et al. 2019). In particular, prolonged dry weather with air temperature ca. > 20 °C has been indicated to accelerate the flowering onset in Tilia species (Szklanowska and Teper 1999). In fact, the acceleration of the Tilia flowering onset has been observed not only in a short-term period but also in a long-term period. For example, Weryszko-Chmielewska et al. (2019) documented a significant acceleration in the beginning of flowering of Tilia species (by 14 days) over a period of 18 years in SE Poland.
The disadvantageous feature of all the studied Tilia species was the short duration of their flowering, i.e. only 10–14 days. A short flowering period in linden species (T. cordata and T. tomentosa, only 12–14 days) was also reported by Pawlikowski (2010). However, the food availability can be extended by strategic selection of different Tilia species in urban ornamental planning. We assume that the food supply period can be prolonged up to ca. 8 weeks if the species and cultivars studied here are included in complex plant arrangements. As shown by Daniels et al. (2020), if properly chosen, Tilia species can fill the late spring/the early summer ‘food gap’ and support food continuity in the urban landscape for more than a month in Central/Western Europe.
Food resource potential
Not surprisingly, we found that the Tilia species differed in floral reward traits (nectar amount, nectar sugar concentration, nectar sugar proportion, and pollen production). Generally, Tilia species are classified among very good nectar-yielding plants (e.g. Farkas and Zajácz 2007; Sun et al. 2020; Sultanova et al. 2022). In our study, T. tomentosa “Petiolaris’, T. × euchlora, T. americana, T. platyphyllos, and T. dasystyla were the top nectar and sugar producers. In every year of our investigations, T. amurensis was a particularly poor nectar-producing species (only 0.31–0.94 mg of sugars per flower). This may indicate that the species is sensitive to some conditions of the urban environment, as it produced high amounts of nectar (c.a. 3.58 mg per flower) in Korea, where it is a native species (Kim et al. 2014; Adgaba et al. 2017 and references therein). However, other causes of such disparity are also possible (e.g. the methods of measurements). The year-to-year variability in nectar/sugar amount (even 2–threefold) was also shown in other linden species. As shown in several earlier studies, nectar secretion in Tilia flowers is extremely unstable in time and may vary considerably between days and even throughout the same day (Jabłoński and Kołtowski 1999; Illies and Muehlen 2007).
The nectar sugar concentration was > 30%, which classifies lindens among pollinator-attractive species (Nicolson 2011; Kim et al. 2014; Ion et al. 2018). However, we found that nectar was highly concentrated (e.g. > 70%—T. platyphyllos in 2018 or T. amuriensis in 2019), which may make it difficult to collect by pollinators. Other researchers also pointed out that, in some years, the nectar of Tilia species may be too concentrated or too diluted and may thus be avoided as unattractive (e.g. Jabłoński and Kołtowski 1999).
Various authors propose that the amount of sugars produced should be taken into account when selecting species to enhance pollinator food resources (Baldock 2020; Tew et al. 2021). This recommendation is related to the high energy demands of insects. For example, one honey bee requires ca. 41.5 mg of nectar/day (Rodney and Purdy 2020). Therefore, 4–8 nectar-rich or ca. 25 nectar-poor flowers must be foraged in order to meet the daily energy needs of individual honey bees.
In addition to the nectar sugar variability in the flowers, our study showed considerable differences in pollen production per flower between the Tilia species. The pollen production in the flowers was related to the interspecies differences in the number of developed anthers. The variability in pollen production in flowers may also be associated with the size of anthers, as documented by Denisow (2011) or Dmitruk et al. (2022), but this trait was not considered in the current study.
An important feature when evaluating the value of woody species as food resources for insects is the total yield of resources (sugars and/or pollen) available per unit area of tree/shrub crown (e.g. Jabłoński and Kołtowski 1999; Farkas and Zajácz 2007; Dmitruk et al. 2022). In view of the total nectar sugar production per 1m2 of tree crown in the Tilia species studied, we in particular recommend T. platyphyllos and T. × euchlora. Additionally, T. americana and T. tomentosa ‘Petiolaris’ are valuable and ensure energy resources for insect visitors in urban green spaces. However, it has to be stressed that the yield of available sugars fluctuates considerably between years. Year-to-year total sugar yield variations are derivative of the differences in the number of developed flowers, which was emphasized by Jabłoński and Kołtowski (1999). For example, in Tilia cordata, they reported considerable differences in the number of developed flowers (from 121 000 to 1, 473 000 flowers per tree) and fluctuations in total sugars (from 0.3 to 2.0 kg per tree) between seasons. Therefore, a significant decrease in the available energy resources derived from linden trees can be expected in some years. In extreme conditions (e.g. short and weak blooming, poor nectar secretion, excessively concentrated or diluted nectar), the linden honey harvest is limited (Farkas and Zajácz 2007; Vercelli et al. 2021) and even starvation of wild pollinators (bumblebees) is observed in areas with a high local density of linden trees and a lack of alternative nectar sources during their blooming (Illies and Muehlen 2007). Hence, the instability of linden resources has to be taken into consideration by urban green planners. For example, the introduction of annual/perennial plants in the vicinity of linden trees to supplement possible nutritional deficiencies during the period of poorer linden nectar production should be considered.
Regarding the total pollen yield per 1 m2 of tree crown and the similar pollen value (measured by the protein content in pollen), we mainly propose T. platyphyllos and T. × euchlora as high-pollen-producing species. However, the other species are also valuable, given the considerable decline in pollen resources during the summer period in various landscape types (e.g. Timberlake et al. 2019; Baldock 2020). As in the case of sugar resources, pollen resources may vary considerably among years. In general, the high annual fluctuations in pollen resources in woody species are related to alternating flowering, which was emphasized previously (e.g. Szklanowska and Teper 1999; Dmitruk 2019; Sultanova et al. 2022).
The estimation of the amount of resources (nectar sugars and/or pollen) in individual trees is essential for reasonable management and improvement of nectar and pollen resources in urban areas. In general, precise evaluation of food resources available in trees is difficult, as the trait depends on many different variables (crown shape, tree age, resources per flower, abundance of flowering). In this study, we calculated the amount of sugar and pollen per 1 m2 as the first step necessary for the assessment of the availability of resources per tree. Having average sugar/pollen yield data calculated for 1 m2 of crown surface, one can estimate the total resources per tree using the data on the crown surface area. In Poland, the crown surface data of different trees can be easily obtained from the MapaDrzew™ application (https://www.mapadrzew.com/).
In view of the nectar carbohydrate composition, the nectar in most Tilia species is sucrose rich (Nicolson and Thornburg 2007), with a sucrose/hexose ratio in the range of 0.5–1.0 and sucrose content between 26 and 50%. Sucrose-dominant nectar was produced by T. × euchlora (sucrose/hexose ratio > 1; sucrose content 51–100%). Similarly, only three types of sugars were detected by Baal (1994) or Somme et al. (2016), who analyzed the carbohydrate profiles in different Tilia species (T. tomentosa, T. platyphyllos, T. cordata, T. × europea, T. × euchlora). No mannose carbohydrate was found in the nectar of Tilia species in the present study. Mannose was regarded to be a toxic component in linden nectars by Madel (1977) and Pawlikowski (2010), who even found dead bees under linden trees. However, based on the results of multiple scientific studies, Koch and Stevenson (2017) indicated that there was no evidence for Tilia nectar toxicity. We recorded diverse insect visitors (mainly honey bees but also bumble bees, solitary bees, and flies) collecting nectar and pollen in the Tilia species, but we did not observe any dead insects under the trees or in the surrounding area. Nevertheless, Tilia nectar still needs investigations towards evaluation the possible presence of chemicals (e.g. nicotine) that are considered toxic (Singaravelan et al. 2006; Fossen et al. 2018; Lande et al. 2019).
Regarding the pollen qualitative value, we found low protein content in the Tilia species compared to other tree species, e.g. Prunus > 20% (Prđun et al. 2022), Aesculus carnea (89.8%), or Acer pseudoplatanus (71.0%) (Somme et al. 2016). In general, the quantity of protein positively correlates with the health and development of bee larvae, and pollen with > 20% protein content is considered the best (Vanderplanck et al. 2014). However, other bioactive compounds (e.g. sterols, vitamins) and micro- and macroelements contained in pollen are necessary in the pollinator diet. Therefore, a diversity of plant species is recommended in urban arrangements to cover the nutritional requirements of pollinators (e.g. Filipiak et al. 2017; Daniels et al. 2020; Baldock 2020). There is also a need for evaluation of pollen quality in as many plant species as possible in the future.
Insect visitors
In agreement with other research (e.g. Anderson 1976; Pawlikowski 2010), the linden flowers in our study region were visited by honeybees, bumblebees, solitary bees, and flies. As in the study conducted by Jacquemart et al. (2018), honey bees dominated the linden flowers. However, we found that the frequency of honey bees differed between the Tilia species. Moreover, the distribution of honey bees for particular linden trees varied among the study years. In our study, the predominance of honey bees can be related to the presence of urban apiaries within the area where the examined trees were grown (see Fig. 1). As reported by Jacquemart et al. (2018), honey bees are constant visitors to linden flowers regardless of whether there are apiaries nearby. Surprisingly, we found that T. amuriensis (poor sugar and pollen producer) was willingly visited by honey bees, although in only one study year. It is accepted that insect assemblage and frequency to particular plant species are related to multiple factors (floral reward amount, chemistry of nectar and pollen, floral volatiles). Moreover, local insect pollinator diversity and density determine their abundance and visitation pattern to flowering plants (Rollings and Goulson 2019). In addition, various abiotic and biotic factors (co-flowering plans, competition between insect groups) can potentially modify plant-pollinator relationships (Illies and Muehlen 2007; Garbuzov and Ratnieks 2014; Ghosh et al. 2020). The attractiveness of flowers to insects is complex and still poses multiple challenges in the elucidation of the variability of spatio-temporal interactions and mechanisms of the effects of floral resources on insect visitor assemblages and frequencies in individual Tilia species.
In conclusion, in the temperate zone, Tilia trees can support insect pollinators with both nectar and pollen mainly in June/July. In particular, T. × euchlora and T. platyphyllos should be considered for the improvement of food resources. In conservation schemes, high year-to-year fluctuations of total sugar and pollen resources should be taken into account. Therefore, landscape managers ought to consider the introduction of other flowering plants with more stable availability of sugars and pollen to cover the food requirements of insects during the period of temporary shortages/lack of nectar sugars/pollen in linden trees. Diverse pollinators (honey bees, bumble bees, solitary bees, and flies) can benefit from Tilia species. Linden trees provide also other environmental benefits in urban areas (e.g. pollutant absorption, improvement of microclimate, reduction of heat island effects) as well as esthetic values.
Data availability
The raw data are available from the corresponding author upon the reasonable request.
References
Adgaba N, Al-Ghamdi A, Tadesse Y et al (2017) Nectar secretion dynamics and honey production potentials of some major honey plants in Saudi Arabia. Saudi J Biol Sci 24:180–191. https://doi.org/10.1016/j.sjbs.2016.05.002
Anderson GJ (1976) The Pollination Biology of Tilia. Am J Bot 63:1203–1212. https://doi.org/10.2307/2441737
Ayers AC, Rehan SM (2021) Supporting bees in cities: how bees are influenced by local and landscape features. InSects 12:128
Baldock KC (2020) Opportunities and threats for pollinator conservation in global towns and cities. Curr Opin Insect Sci 38:63–71. https://doi.org/10.1016/j.cois.2020.01.006
Baude M, Kunin WE, Boatman ND et al (2016) Historical nectar assessment reveals the fall and rise of floral resources in Britain. Nature 530:85–88
Bayer D, Reischl A, Rötzer T, Pretzsch H (2018) Structural response of black locust (Robinia pseudoacacia L.) and small-leaved lime (Tilia cordata Mill.) to varying urban environments analyzed by terrestrial laser scanning: Implications for ecological functions and services. Urban for Urban Green 35:129–138. https://doi.org/10.1016/j.ufug.2018.08.011
Bernardello G (2007) A systematic survey of floral nectaries. Springer, Nectaries and nectar, pp 19–128
Borys T Markowska M Kobiela K et al 2021 Ranking of Sustainable Polish Cities Ranking Polskich Miast Zrównoważonych
Bożek M (2021) Nectar production and spectrum of insect visitors in six varieties of highbush blueberry (Vaccinium corymbosum L) in SE Poland. Acta Agrobot 74:1
Bożek M, Denisow B, Strzałkowska-Abramek M et al (2023) Non-forest woody vegetation: a critical resource for pollinators in agricultural landscapes—a review. Sustainability 15:8751. https://doi.org/10.3390/su15118751
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Braman SK, Griffin B (2022) Opportunities for and impediments to pollinator conservation in urban settings: a review. J Integr Pest Manag 13:6
Chen D, Chen HW (2013) Using the Köppen classification to quantify climate variation and change: An example for 1901–2010. Environ Dev 6:69–79. https://doi.org/10.1016/j.envdev.2013.03.007
Corbet SA, Unwin D, Prŷs-Jones OE (1979) Humidity, nectar and insect visits to flowers, with special reference to Crataegus, Tilia and Echium. Ecol Entomol 4:9–22
Crailsheim K, Schneider L, Hrassnigg N et al (1992) Pollen consumption and utilization in worker honeybees (Apis mellifera carnica): dependence on individual age and function. J Insect Physiol 38:409–419
Czaja M, Kołton A, Muras P (2020) The complex issue of urban trees—Stress factor accumulation and ecological service possibilities. Forests 11:932
Daniels B, Jedamski J, Ottermanns R, Ross-Nickoll M (2020) A “plan bee” for cities: Pollinator diversity and plant-pollinator interactions in urban green spaces. PLoS ONE 15:e0235492
Denisow B (2011) Pollen production of selected ruderal plant species in the Lublin area. WUP Wydawnictwo Uniwersytetu Przyrodniczego Lublin, Poland
Di Pasquale G, Alaux C, Le Conte Y et al (2016) Variations in the availability of pollen resources affect honey bee health. PLoS ONE 11:e0162818
Dmitruk M (2019) Flowering, nectar secretion, and structure of the nectary in the flowers of Acer pseudoplatanus L. Acta Agrobot 72:3
Dmitruk M, Strzałkowska-Abramek M, Bożek M, Denisow B (2022) Plants enhancing urban pollinators: Nectar rather than pollen attracts pollinators of Cotoneaster species. Urban for Urban Green 74:127651. https://doi.org/10.1016/j.ufug.2022.127651
Donkersley P (2019) Trees for bees. Agric Ecosyst Environ 270–271:79–83. https://doi.org/10.1016/j.agee.2018.10.024
Mapa DrzewTM - informacje o drzewach w Polsce. https://mapadrzew.com. Accessed 1 Mar 2024b
Farkas Á, Zajácz E (2007) Nectar production for the Hungarian honey industry. Eur J Plant Sci Biotechnol 1:125–151
Filipiak M (2019) Key pollen host plants provide balanced diets for wild bee larvae: a lesson for planting flower strips and hedgerows. J Appl Ecol 56:1410–1418
Filipiak M, Kuszewska K, Asselman M et al (2017) Ecological stoichiometry of the honeybee: Pollen diversity and adequate species composition are needed to mitigate limitations imposed on the growth and development of bees by pollen quality. PLoS ONE 12:e0183236
Fossen T, Holmelid B, Øvstedal D (2018) Bumblebee death associated with Tilia x europaea L. Biochem Syst and Ecol 82:16–23. https://doi.org/10.1016/j.bse.2018.11.001
Garbuzov M, Ratnieks FL (2014) Quantifying variation among garden plants in attractiveness to bees and other flower-visiting insects. Funct Ecol 28:364–374
Geslin B, Gauzens B, Thébault E, Dajoz I (2013) Plant pollinator networks along a gradient of urbanisation. PLoS ONE 8:e63421
Ghosh S, Jeon H, Jung C (2020) Foraging behaviour and preference of pollen sources by honey bee (Apis mellifera) relative to protein contents. Journal of Ecology and Environment 44:1–7
Glen HF (2002) Cultivated plants of Southern Africa. Johannesburg, Jacana
Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:1255957
Grote R, Samson R, Alonso R et al (2016) Functional traits of urban trees: air pollution mitigation potential. Front Ecol Environ 14:543–550. https://doi.org/10.1002/fee.1426
Hall DM, Camilo GR, Tonietto RK et al (2017) The city as a refuge for insect pollinators. Conserv Biol 31:24–29
https://pl.climate-data.org. https://pl.climate-data.org/. Accessed 18 Mar 2024a
https://www.iqair.com. https://www.iqair.com/. Accessed 18 Mar 2024
Ignatieva M (2011) Plant Material for Urban Landscapes in the Era of Globalization: Roots, Challenges and Innovative Solutions. John Wiley & Sons Ltd, Applied Urban Ecology, pp 139–151
Illies I, Muehlen W (2007) The foraging behaviour of honeybees and bumblebees on late blooming lime trees (Tilia spec) (Hymenoptera: Apidae). Entomol Gen 30:155–165
Ion N, Odoux J-F, Vaissière BE (2018) Melliferous Potential of Weedy Herbaceous Plants in Crop Fields of Romania from 1949 to 2012. J Apic Sci 62:149–165
Jablońska K, Kwiatkowska-Falinska A, Czernecki B, Walawender J (2015) Changes in spring and summer phenology in poland — responses of selected plant species to air temperature variations. Pol J Ecol 63:311–319. https://doi.org/10.3161/15052249PJE2015.63.3.002
Jabloński B (2002) Notes on the method to investigate nectar secretion rate in flowers. J Apic Sci 46(2):117–124
Jabłoński B, Kołtowski Z (1999) Nektarowanie roznych gatunkow i mieszancow lipy [Tilia L.]. Pszczelnicze Zeszyty Naukowe 43:279–290
Jacquemart A-L, Moquet L, Ouvrard P et al (2018) Tilia trees: toxic or valuable resources for pollinators? Apidologie 49:538–550. https://doi.org/10.1007/s13592-018-0581-3
Kim MS, Kim SH, Song JH, Kim H (2014) Analysis of secreted nectar volume, sugar and amino acid content in male and female flower of Evodia daniellii Hemsley. J. Korean for. Soc. 103:43–50. https://doi.org/10.14578/jkfs.2014.103.1.43
Koch H, Stevenson PC (2017) Do linden trees kill bees? Reviewing the causes of bee deaths on silver linden (Tilia tomentosa). Biol Lett 13:20170484
Kusiak W, Majka J, Zborowska M, Ratajczak I (2022) Chemical composition and related properties of lime (Tilia cordata Mill) bark and wood as affected by tree growth conditions. Materials 15:4033. https://doi.org/10.3390/ma15114033
Lande C, Rao S, Morré JT et al (2019) Linden (Tilia cordata) associated bumble bee mortality: metabolomic analysis of nectar and bee muscle. PLoS ONE 14:e0218406. https://doi.org/10.1371/journal.pone.0218406
Li D, Sullivan WC (2016) Impact of views to school landscapes on recovery from stress and mental fatigue. Landsc Urban Plan 148:149–158
Madel G (1977) Vergiftungen von Hummeln durch den Nektar der Silberlinde Tilia tomentosa Moench. Bonn Zool Beitr. 28:149–154
Masierowska M, Stawiarz E, Rozwałka R (2018) Perennial ground cover plants as floral resources for urban pollinators: a case of Geranium species. Urban for Urban Green 32:185–194
Nicolson SW (2011) Bee food: the chemistry and nutritional value of nectar, pollen and mixtures of the two. Afr Zool 46:197–204
Nicolson SW, Thornburg RW (2007) Nectar chemistry. Springer, Nectaries and nectar, pp 215–264
Nowak DJ, Hirabayashi S, Doyle M et al (2018) Air pollution removal by urban forests in Canada and its effect on air quality and human health. Urban for Urban Green 29:40–48
Pawlikowski T (2010) Pollination activity of bees (Apoidea: Apiformes) visiting the flowers of Tilia cordata Mill. and Tilia tomentosa Moench in an urban environment. J Apic Sci 54:73–79
Phillips BB, Wallace C, Roberts BR et al (2020) Enhancing road verges to aid pollinator conservation: A review. Biol Conserv 250:108687
Popek R, Gawrońska H, Wrochna M et al (2013) Particulate Matter on Foliage of 13 Woody Species: Deposition on Surfaces and Phytostabilisation in Waxes – a 3-Year Study. Int J Phytoremediation 15:245–256. https://doi.org/10.1080/15226514.2012.694498
Prđun S, Flanjak I, Svečnjak L et al (2022) Characterization of rare Himalayan Balsam (Impatiens glandulifera Royle) honey from croatia. Foods 11:3025. https://doi.org/10.3390/foods11193025
Radoglou K, Dobrowolska D, Spyroglou G, Valeriu-Norocel N (2009) A review on the ecology and silviculture of limes: (Tilia cordata Mill., Tilia platyphyllos Scop, and Tilia tomentosa Moench.) in Europe. Bodenkultur -Wien and Munchen 3:9–20
Rodney S, Purdy J (2020) Dietary requirements of individual nectar foragers, and colony-level pollen and nectar consumption: a review to support pesticide exposure assessment for honey bees. Apidologie 51:163–179. https://doi.org/10.1007/s13592-019-00694-9
Rollings R, Goulson D (2019) Quantifying the attractiveness of garden flowers for pollinators. J Insect Conserv 23:803–817
Roulston TH, Cane JH (2000) Pollen nutritional content and digestibility for animals. Plant Syst Evol 222:187–209
Sadowiec KJ, Gawroński SW (2013) Przydatność wybranych gatunków lip (Tilia sp) do fitoremediacji powietrza z zanieczyszczeń pyłowych. Wiejskie T, Woda-Środowisko-Obszary
Seneta W Dolatowski J 2012 Dendrologia [Dendrology]. Warszawa: Wyd Nauk PWN [in Polish] 544
Singaravelan N, Inbar M, Ne’eman G, et al (2006) The effects of nectar-nicotine on colony fitness of caged honeybees. J Chem Ecol 32:49–59. https://doi.org/10.1007/s10886-006-9350-2
Somme L, Vanderplanck M, Michez D et al (2015) Pollen and nectar quality drive the major and minor floral choices of bumble bees. Apidologie 46:92–106
Somme L, Moquet L, Quinet M et al (2016) Food in a row: urban trees offer valuable floral resources to pollinating insects. Urban Ecosyst 19:1149–1161. https://doi.org/10.1007/s11252-016-0555-z
Sultanova R, Martynova M, Sazgutdinova R (2022) Honey-Bearing Potential of Tilia cordata Mill. Forests in the Southern Urals. Front Ecol Evol 10:2022. https://doi.org/10.3389/fevo.2022.832442
Sun Z, Liu Q-J, Xu Z-Z, Xu W-Z (2020) Nectar productivity of Tilia amurensis in a broadleaved-conifer mixed forest in Changbai Mountains, China. Chin J Appl Ecol 31:2500–2506
Szklanowska K, Teper D (1999) Wydajnosc pylkowa roznych gatunkow i mieszancow lipy [Tilia L.]. Pszczelnicze Zeszyty Naukowe 43:291–302
Szklanowska K, Teper D, Jabłoński B, Kołtowski Z (1999) Wybrane zjawiska biologii kwitnienia roznych gatunkow i mieszancow lipy [Tilia L.] oraz oblotu ich przez pszczoly. Pszczelnicze Zeszyty Naukowe 43:263–278
Tew NE, Memmott J, Vaughan IP et al (2021) Quantifying nectar production by flowering plants in urban and rural landscapes. J Ecol 109:1747–1757
Theodorou P, Radzevičiūtė R, Lentendu G et al (2020) Urban areas as hotspots for bees and pollination but not a panacea for all insects. Nat Commun 11:576
Timberlake TP, Vaughan IP, Memmott J (2019) Phenology of farmland floral resources reveals seasonal gaps in nectar availability for bumblebees. J App Ecol 56:1585–1596
Ulrich RS, Simons RF, Losito BD et al (1991) Stress recovery during exposure to natural and urban environments. J Environ Psychol 11:201–230
Vanderplanck M, Moerman R, Rasmont P et al (2014) How Does Pollen Chemistry Impact Development and Feeding Behaviour of Polylectic Bees? PLoS ONE 9:e86209. https://doi.org/10.1371/journal.pone.0086209
Vaudo AD, Tooker JF, Grozinger CM, Patch HM (2015) Bee nutrition and floral resource restoration. Curr Opin Insect Sci 10:133–141
Vercelli M, Novelli S, Ferrazzi P et al (2021) A Qualitative Analysis of Beekeepers’ Perceptions and Farm Management Adaptations to the Impact of Climate Change on Honey Bees. InSects 12:228. https://doi.org/10.3390/insects12030228
Weryszko-Chmielewska E, Piotrowska-Weryszko K, Dąbrowska A (2019) Response of Tilia sp. L. to climate warming in urban conditions – Phenological and aerobiological studies. Urban for Urban Green 43:126369. https://doi.org/10.1016/j.ufug.2019.126369
Acknowledgements
We would like to deeply thank two anonymous Reviewers for their valuable comments on the first version of the manuscript. Our thanks also go to Urszula Bronowicka-Mielniczuk PhD for the assistance with statistical analyses. We would like to thank Monika Strzałkowska-Abremek, PhD for the assistance during the field studies, laboratory procedures and assistance in data visualization
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hormaza.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dmitruk, M., Denisow, B., Chrzanowska, E. et al. Comparison of nectar and pollen resources in various Tilia species. A case study from southern Poland. Trees (2024). https://doi.org/10.1007/s00468-024-02527-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00468-024-02527-4