Key message
This compilation is focused on the role of cork oak periderm, a protective layer with unique physical and chemical traits for the cork industry, highlighting the recent periderm-specific genomic resources available.
Abstract
Cork oak is a unique species with the ability to produce a continuous and renewable cork throughout its lifespan. Periderm is a protective tissue composed of the phellem, phellogen, and phelloderm that replaces the epidermis. Phellem or “cork”, the outermost layer, is produced by the original phellogen, a secondary meristem originated from the dedifferentiation of mature parenchyma cells. The formation and differentiation of periderm have been widely studied demonstrating the importance of fatty acid biosynthesis, phenylpropanoid, and metabolism of suberin, a complex glycerol-based polymer and the principal component of phellem. The contributions of several areas reveal new clues concerning the molecular mechanisms behind periderm differentiation. However, the whole process is still poorly understood. In this review, we compile information regarding the cellular structure and molecular basis, including the regulatory network of periderm formation and differentiation, focusing on the cork oak. The cork quality and its genetic and epigenetic mechanisms are also explored, highlighting the importance of molecular regulation in such economically important species. An increased understanding of the all periderm differentiation process may serve as a basis for future studies on functional genomics with an impact on fundamental science and on the forest industry for the production of high-quality cork.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The periderm is an outer defensive secondary tissue in woody plants that continuously replaces epidermal tissue near the surface of stems and roots. In most gymnosperms and woody dicots, this replacement generally occurs at the end of the first year, like in cork oak, or during the beginning of the second year of growth (Graça and Pereira 2004; Crang et al. 2018; Coder 2019). The periderm is composed of three tissues from outward to interior: the phellem, phellogen, and phelloderm. These layers serve as a means of protection against adverse environmental conditions, such as temperature variation and water loss, fire-damage, and biological attack (e.g., insects, fungi, etc.) due to its specialized cellular structure and chemical composition (Dickison 2000; Pereira 2015; Heldt and Piechulla 2021). Phellem, the outermost layer, commonly designated as “cork”, is produced from periclinal divisions of the original phellogen, a secondary meristem, by a process of differentiation. This process includes cell expansion, suberization of cell walls, deposition of waxes, and cell death (Natividade 1950; Graça and Pereira 2004). Some species of trees, like Quercus suber L. (Cork oak), Quercus variabilis Blume (Chinese cork oak), and Phellodendron amurense Rupr. (Amur cork tree), have the unique ability to produce a continuous and renewable cork (Leite and Pereira 2017). However, only cork of Quercus suber has physical, chemical, and mechanical properties desirable for industrial application of high-quality (Natividade 1950; Leite and Pereira 2017). The continuous regeneration of the phellogen in cork oak enables the harvesting of cork for commercial purposes, every 9–12 years, after the first extraction, without debilitating the trees (Oliveira and Costa 2012; Louro et al. 2014). The chemical properties of the phellem have been well studied in Q. suber (Pereira 1988; Costa et al. 2019). These properties have also been thoroughly examined in potato (Solanum tuberosum L.) (Graça and Pereira 2000). Potato has been considered a good model for studies related to suberin biosynthesis and structure of periderm, because of its secondary suberization induced by wound and easier isolation of periderm from the tubers for chemical analyses (Jin et al. 2018; Woolfson et al. 2018, 2022; Serra and Geldner 2022). In addition, the relatively more rapid growth compared to trees, and easy in vitro transformation for gene function studies, also assists such studies (Sabba and Lulai 2002; Serra et al. 2009a, b; Soler et al. 2011, 2020). However, a full understanding of how suberin, other macromolecules of phellem cell walls, and regulation of molecular mechanisms involved in periderm formation is still a challenge. This review is intended to cover the molecular basis of periderm formation and differentiation, focusing on a case study of cork oak, a unique cork producer species, highlighting the genetic and epigenetic mechanisms of cork quality, and the process behind suberin biosynthesis and its role as the main component of cork tissue.
Formation and differentiation of periderm
The phellogen differentiates in the first years of growth not only in the epidermis, hypodermis, or phloem in most trees stem, but also in roots where it derives from the pericycle (Machado et al. 2013; Wunderling et al. 2018; Macnee et al. 2020; Andersen et al. 2021; Leal et al. 2022a). However, in some species, such as carob (Ceratonia siliqua L.) (Arzee et al. 1977) and box elder (Acer negundo L.) (Wacowska 1985) the first continuous periderm is formed in stems only in the sixth year. The formation of the phellogen results from the dedifferentiation of mature parenchyma cells (i.e., return to a meristematic function) by periclinal division. Thus, the meristematic activity of phellogen originates the production of phelloderm (inside) and phellem cells (outside) (Fig. 1) (Graça and Pereira 2004; Leite and Pereira 2017; Crang et al. 2018; Serra et al. 2022). Normally, only one layer of phelloderm is produced, in contrast to several layers of phellem (Beck 2010; Leite and Pereira 2017). Division of the phellogen occurs only after the suberization of the previously divided cells. Complete differentiation of the maturing phellem cell appears to precede the division by the phellogen. This process is induced by the necessity for protection afforded by the suberized cell walls (Graça and Pereira 2004). The activity of the phellogen is linked not only to genetic regulation, but also to physiological and environmental constraints including availability of water, temperature, oxidative stress due to sun irradiation, mechanical or biotic wounding, and aging (Lev-Yadun 2011).
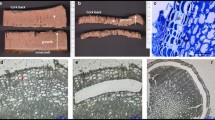
Quercus suber tissues from a 1-year-old a and 3-year-old b, c branches. Phellem or cork (C); cork cambium or phellogen (Ph); cortex (Cx); lenticel (L) and lenticular phellogen (Lp). The histological analysis was performed using cryosectioning tissues previously flash-frozen, according to Pires et al. 2022a . Scale bars 50 μm
A recent study in Arabidopsis thaliana L. identified six stages for periderm development in roots (Wunderling et al. 2018; Campilho et al. 2020). The development started with the division of pericycle (stage 1) followed by programmed cell death (PCD) and periclinally division by pericycle (stage 2). In stage 3/4, the cortex and epidermis break, and the phellem is already differentiated, and suberized. In stage 5, the epidermis and outer cortex break, and progressively become detached, and finally, in the last stage, the periderm is already developed (Wunderling et al. 2018; Campilho et al. 2020). In the differentiation process shown for Arabidopsis, two independent mechanisms are involved: PCD of the outer tissues (stage 2) and abscission of the cortex and epidermis (stage 3/4) (Wunderling et al. 2018). Also, it was demonstrated in Arabidopsis that suberization is initiated early after phellogen establishment and cell division (stages 2–3/4) by a combined strategy of monitorization of suberin markers genes activation and anatomical analyses (Leal et al. 2022a).
In the roots of cork oak, young periderm develops from pericycle cells under the endodermis, like in Arabidopsis. A two/three-layer ring of suberized cells (phellem) is filled with phenolic compounds pushed outward the remained cortex and epidermal layers, becoming the outermost tissue of mature roots (Machado et al. 2013). The study of spatiotemporal development of suberized cork oak taproots, demonstrated new periclinal cell divisions between xylem and phloem tissues indicating the establishment of the vascular cambium, at 6 days after sowing (DAS), while the phellogen specification was detected at 8 DAS. At 15 DAS, an extensive vascular cambium activity was pointed, while the suberin was detected in one to two rows of cells showing the existence of phellem cells. At 22 DAS and 60 DAS the continuous activity of phellogen with the presence of a multilayered suberized phellem was revealed (Leal et al., 2022b).
The cork oak tree
Cork oak (Quercus suber; Section Cerris) is an evergreen oak tree widespread throughout the Mediterranean region, having a climate characterized by hot, dry, and sunny summers, mild winters, and rainy autumns (Lavorel et al. 1998; Soler et al. 2008) (Fig. 2a). It is mainly distributed within the Iberian Peninsula, covering 715 923 ha in Portugal and 500 000 ha in Spain (Aronson et al. 2009; Duque-Lazo et al. 2018) corresponding to more than 50% of the world’s distribution (Oliveira and Costa 2012). Cork oak stands are unique dynamic ecosystems representing some of the world’s most biodiverse “hotspots” (Gil 2014; Simonson et al. 2018), whose natural habitat was included in the special areas of conservation, under the European Community Habitats Directive (Council Directive 92/43/EEC on the Conservation of wild habitats and of wild fauna and flora).
Besides the relevant ecological and social importance of this keystone species, it has also a great economic value, mainly in the production of commercial cork. The outer bark of cork oak can be periodically harvested for commercial cork (Fig. 2b), while the tree maintains the unique ability to regenerate new layers of phellem resulting in the production of cork repeatedly, up to a dozen or more times throughout its lifespan, often exceeding two centuries (Pereira and Tomé 2004). The first cork to be harvested, referred to as virgin cork, is typically harvested after 19–25 years of tree growth when the tree reached a legal size of 70 cm perimeter at breast height in Portugal (Oliveira and Costa 2012). After removal of the cork, the underlying phellogen dies, and a new traumatic phellogen is formed by a process of meristematic activation (Fortes et al. 2004). The following cork extraction (Fig. 2b), after the first harvesting, usually occurs every 9–12 years, when cork reaches a thickness of 2–6 cm (Silva et al. 2005).
New cultural conditions involving irrigation systems have recently been applied to cork oak plantations. Irrigation results in more rapid tree growth and achievement, shortening time to 6–10 years for first bark-stripping, with possible potential economic benefits (Vessella et al. 2010; Camilo-Alves et al. 2020). However, the quality of cork harvested under these cultural conditions needs further evaluation.
Cork’s structure has an inherent heterogeneity given by the formation of the annual rings that represent the yearly growth rhythm of cork (Pereira and Tomé 2004). Cork is only harvested between mid-spring (late May) and summer (mid-August) when the phellogen is at maximal physiological activity and is swollen. Because the newly formed cells have thin and fragile cell walls, still not suberized, they are easily ruptured, facilitating separation of the cork from the underlying tissues (Oliveira and Costa 2012). The tree diameter and cork growth are affected by annual weather variations. Precipitation during the winter months enhances cork growth, between March and October, by ensuring that soil water is available to initiate the next growth period. Low temperatures during the winter months when the phellogen is inactive, generally hinder growth (Costa et al. 2003). In addition to climate or water availability, recurrent harvesting may influence the radial growth, in terms of cork and wood increments. In cycles of 9–12 years, the cork thickness particularly increases in the first 2–4 years after harvesting, representing the majority of the whole cork production cycle (Oliveira et al. 2002; Oliveira and Costa 2012). Thus, the monthly growth rates regularly decrease until the end of the cycle possibly as a result of a decline in meristematic activity, and the of cell production by phellogen (Pereira et al. 1992). However, stripping may negatively affect vascular cambium activity. A decrease in wood growth was observed in the years after cork harvesting, even under favorable water availability conditions. (Leal et al. 2008). The cork harvesting may affect the tree energy balance for months and years after stripping, having an impact on growth and reproduction of cork oak (Oliveira and Costa 2012). Some authors indicate that tree vigor breakpoint can occur at the sixth consecutive harvest (Costa et al. 2015; Leite and Pereira2017). Cork harvesting can also be stressful for the tree, decreasing the growth rate of flowers, fruits, and roots (Chapin et al.2003; Costa et al. 2015). Moreover, factors like bark thickness and fire events after stripping can influence post-fire tree survival (Moreira et al. 2007). Inadequate or extensive/over-exploitation stripping may also lead to a gradual reduction of tree’s vigor or longevity (Oliveira and Costa 2012), highlighting the importance of good management practices on cork oak woodlands.
The cork cells
The phellem of cork trees, commercial cork, is characterized by a deposition of layered, dead suberized cells, composed of hexagonal prism cells (height 30–40 μm with cell wall thickness 1–1.5 μm) stacked by their bases in rows radiating outwards from the phellogen, disposed in parallel, with no intercellular spacing (Pereira and Tomé 2004). In tissue cross sections, cork cells appear as a honeycomb structure. In longitudinal or radial sections, the cells depict a brick-wall like structure (Leite and Pereira 2017). The cellular structure and the chemical composition are responsible for the main physical and mechanical properties of cork, such as low thermal coefficient, very low permeability to fluids, low density, buoyancy, and elasticity (Miranda et al. 2013; Pereira 2015). Structural matrix polymers of the cork cell wall consist of suberin, representing 53% of the structural components, lignin 26%, cellulose 10%, and hemicellulose representing approximately 11%. We can also identify an appreciable amount of extractives that include both non-polar and polar compounds (Pereira 2015). During cork cells differentiation, suberin deposition has been described as one of the main internal processes occurred in the internal side of cell walls (Graça and Pereira 2004). Besides the cell wall suberization, the deposition of condensed tannins, proanthocyanidins, is observed in cell lumen or cell walls getting into PCD process (Inácio et al. 2018). This is corroborated by the expression of the main genes of suberin, proanthocyanidin biosynthesis, autophagy, and PCD in differentiating cork periderm described by Inácio et al. (2021).
Molecular and regulatory mechanisms of periderm differentiation
Genomic studies of cork oak identified candidate genes for cork differentiation. With the formation of the consortium to study cork oak expression sequence tags (ESTs), Cork oak ESTs Consortium (COEC), it was possible to conduct the first large-scale sequencing of 21 cDNA libraries. This resulted in the construction of the first cork oak transcriptome database, containing 159,000 unigenes, providing an important step to reveal the molecular cascades underlying cork differentiation (Pereira-Leal et al. 2014). Following the construction of this database, the first draft version of the cork oak genome was produced by a de novo assembly strategy based on high-throughput sequence data (Ramos et al. 2018). In addition, new periderm transcriptomic studies were also developed (Lopes et al. 2019; Fernández-Piñán et al. 2021; Leal et al. 2022b; Pires et al. 2022a, b). Putative cork regulatory genes identified included MYB [MYB52, MYB102, MYB93, MYB9, RAX3/MYB84 (QsMYB1)], No-Apical-Meristem (NAM: NAC058, NAC92), and WRKY (WRKY43) families (Soler et al. 2007; Lopes et al. 2019; Fernández-Piñán et al. 2021; Leal et al. 2022b). These genes were related to meristem identity and associated with maintenance of phellogenic cells or differentiation into phellem cells (Soler et al. 2007; Fernández-Piñán et al. 2021). The seasonal variation analysis by RT-qPCR in cork tissues of selected genes involved in the suberin biosynthetic pathway (CYP86A1, GPAT, and HCBT), and of regulatory genes from NAM and WRKY families, revealed highest transcript accumulation occurred in June (Soler et al. 2008) a crucial month for cork development. This same study showed that levels of expression of cork structural genes, palmitoyl-acyl carrier protein thioesterase (PATE/FAT), and ferulate-5-hydroxylase (F5H), were correlated with temperature and relative humidity. Genes related to ethylene and jasmonate signaling were also predominant in traumatic differentiation of phellem in cork oak. This process was supported by the presence of the cytochrome P450 CYP82D, which putatively regulates jasmonate synthesis, and apetala 2/ethylene responsive factor (AP2/ERF), which mediate ethylene responses (Lopes et al. 2019).The transcription factor (TF) in Q. suber, QsMYB1, was characterized by Almeida et al. (2013a) to understand its role in cork development. Two transcripts of this TF, QsMYB1.1, and QsMYB1.2, are up-regulated in cork tissues, suggesting that this TF can be related to cork development process with a possible alternative splicing mechanism associated with its regulatory function (Almeida et al. 2013a). In addition, the expression profile changes observed by Almeida et al. (2013b) for the two QsMYB1 splicing variants under drought and heat stresses, suggesting that this TF can be modulated at the transcriptional level under abiotic stresses. A ChIP-Seq study in cork oak also revealed that QsMYB1 directly targets genes involved in lignin and suberin biosynthesis through regulation of phenylpropanoid pathway, as well as genes encoding ATP-binding cassette sub-family G transporters (ABCG) and lipid transfer proteins (LTPs) involved in the transport of monomeric suberin units across the cellular membrane (Capote et al. 2018). The alteration of the major components of suberin in potato periderm was also detected in preliminary results of QsMYB1 overexpression lines (Faustino et al. 2022). The TF KUA1, a MYB-like protein family, was upregulated during expansion growth of the A.thaliana leaf (Lu et al. 2014). In cork oak, this TF was also found more expressed in good cork quality (Mendes et al. 2022). Other members from the MYB superfamily, such as MYB41 and MYB107 were identified in A. thaliana and kiwifruit, suggesting a link to suberin biosynthesis (Kosma et al. 2014; Lashbrooke et al. 2016; Gou et al. 2017; Wei et al. 2020; Shukla et al. 2021).
A comparison of outer bark transcriptomes between Q. suber and Q. ilex L., a non-producer of commercial cork, which shares the same habitat, identified a possible set of candidate genes regulating phellogen activity and phellem formation (Boher et al. 2018). These two Quercus species represent different models of phellem differentiation. Comparing their transcriptome activities, the analysis revealed the greater production of cork, by Q. suber, with up-regulation of genes involved with phenylpropanoid metabolism, suberin metabolism, and fatty acid biosynthesis. Alternatively, genes linked to abiotic stress and chromatin assembly were up-regulated in Q.ilex (Boher et al. 2018). The next-generation sequencing technologies, also allowed the identification of putative regulators of periderm differentiation identified in other species. New candidate TFs, from MYB and NAC-families, were identified in an apple variety characterized by the accumulation of suberin in fruit skin, a russet-exocarp of apple (Malus Mill. sp.), revealing an enhanced expression of suberin genes associated with repression of lignin and cuticle biosynthesis (Legay et al. 2015). In that study, MdMYB93 was identified as differentially expressed in russeted vs. non-russeted apple skins. Also, when these genes were ectopically expressed in Nicotiana benthamiana Domin. leaves, suberin, and lignin-related genes were activated and suberin deposition was promoted (Legay et al. 2016). From the transcriptomic analysis of bark tissues in poplar, transcripts of WRKY families were also abundant in cork, in particular WRKY43 (Rains et al. 2017). Also, the SHORT ROOT-like TF in Populus L., PtSHR2B, appears to be related to the regulation of phellem and periderm formation by modulating cytokinin homeostasis (Miguel et al. 2016). The authors showed that the proportion of bark was increased relative to the wood in PtSHR2B overexpressed plants, speculating that increased cytokinin levels in the bark may result from an increased amount of PtSHR2B transcripts.
Auxin, key regulator of plant growth and development, is also required for phellogen establishment and maintenance in Arabidopsis root, but additional signals, are needed to trigger periderm development (Xiao et al. 2020). Some TF, like Wuschel-related homeobox 4 (WOX4) and KNat1/Brevipedicellus (BP) act downstream of auxin in the periderm, reveling a key importance of these regulators in phellogen activity. However, in cork oak, differential auxin signaling and response in phellem can be trigged out. Lopes et al. (2019) identified 20 differentially expressed AUX/IAA transcriptional regulators, but the majority (18) were down-regulated or absent, and only Auxin Response Factor 18 (ARF) was up-regulated in phellem tissues.
Silencing of StNAC103 in potato promotes the accumulation of suberin in the phellem of tubers (Verdaguer et al. 2016). The overexpression of AtANAC046 promotes suberin biosynthesis in roots of A. thaliana (Mahmood et al. 2019). In addition, AtBP/KNAT1 and AtWOX4 promote phellogen proliferation and loss of function mutant shows a delay in periderm growth in A. thaliana (Xiao et al. 2020). As a complement to the transcriptome analyses, a proteomic study on cork formation in Q. suber identified numerous proteins involved with carbohydrate metabolism, defense, protein folding, stability and degradation, and regulation/signaling between others associated with phellem (Ricardo et al. 2011). The presence of defense proteins (thioredoxin-dependent peroxidase, glutathione-S-transferase, SGT1 protein, cystatin, and chitinases) suggests a strong need for cell protection when phellem is active. Although these two approaches, transcriptomic and proteomic analyses, provided further information on the molecular and genomic basis of cork formation and the biological functions of the phellem, the entirety of the phellem differentiation process is still poorly understood.
Suberin: the main constituent of phellem
Suberin, a biopolymer found in specialized plant cell walls, is the principal component of phellem. It has a fundamental role of serving as a protective barrier between plant cells and the environment in normal or wounded tissues above-ground and/or subterranean plant parts (Gandini et al. 2006). The quantity of suberin present in plants, in general, varies according to plant species, tissue, developmental stage, and response to environmental changes (Harman-Ware et al. 2021). Suberized cells can be found in the stem and root periderms, exodermis, and endodermis, in addition to other specialized tissues, including seed coat, fruit and vegetable skin, and abscission zone (Beisson et al. 2012; Harman-Ware et al. 2021). For example, the periderm of S. tuberosum has a suberin content of ≈ 25% (Graça and Pereira 2000), whereas Q. suber has between 37% (Cordeiro et al. 1998) and 62% (Conde et al. 1999), depending upon the method used for extraction. The suberin, found in other higher plants, has been also characterized for Pseudotsuga menziesii (Mirb.) Franco, Q. cerris L., Q. ilex, Q. robur L., Q. variabilis Blume, P. tremula L., Betula pendula Roth, Castanea sativa Mill., among many more (Holloway 1983; Ferreira et al. 2016a; Leite and Pereira 2017; Sillero et al. 2019; Kumar et al. 2022).
Suberin is a complex glycerol-based polymer of a polyaliphatic polyester linked to phenolic components and embedded waxes (Beisson et al. 2012). Kolattukudy (1980) proposed a structure for suberin in which a cross-linked aromatic subdomain is covalently linked to long-chain diacids and hydroxyacids through ester bonds (Kolattukudy 1980). The principal components of suberin responsible for its polymeric structure include ω-hydroxy acids (having carboxyl groups in the α position and hydroxyl groups in the ω position), and ω,α-dicarboxylic acids (carboxyl groups at both the α and ω positions) (Graça 2015). Glycerol can be isolated from cork by methanolysis, leading to the proposal that suberin is a glyceryl-based polymer of cross-linked dicarboxylic fatty acids with glycerol (Graça and Pereira 1997). Ferulic acid plays an important role in cork by cross-linking suberin structural polymers to lignin (the second leading component of cork) through ether bonds (Marques et al. 2016). Ferulic acid is produced by the phenylpropanoid pathway and is considered the main hydroxycinnamic acid present in the polyphenolic components (Bernards 2002). Besides that, mono-functional fatty acids (alkanoic acids) and fatty alcohols (alkanols) are also commonly found among the depolymerization products of most suberin, but in less quantity (Graça 2015).
Microscopic examination of suberized cell walls reveals three main layers. They are the primary wall, internally, a thicker secondary wall lamellate, medially, and a tertiary cell wall, externally (Fig. 3) (Teixeira and Pereira 2010; Crouvisier-Urion et al. 2019). Transmission electron microscopy reveals a consistent regular ultrastructure of suberin composed of alternating opaque and translucent lamellae, also designated as dark and light lamellae (Serra et al. 2009b; Graça 2015). The light translucent lamellae are mainly composed of aliphatic compounds, while the dark bands are rich in phenolic compounds (Graça and Santos 2007; Teixeira and Pereira 2010). The number of lamellae in suberin can vary depending on the tissue or plant species examined. For example, the number of lamellae of the suberin in the skins of potato tubers is less than that in the cork of Q. suber (Graça 2015).
Periderm in Solanum tuberosum by scanning electron microscopy—SEM (a, b), and transmission electron microscopy TEM (c). (a)—Suberized cells of phellem (Phl), amylaceous parenchyma tissue (Apt); (b)—Outer tissue of periderm and phellem (Phl); (c)—Suberized cells walls consist of primary wall with compound middle lamella (CML), secondary wall (Sec) and tertiary wall with waxes deposited (Ter + wax)
Suberin biosynthesis: molecular regulation
Several studies on understanding the mechanisms of periderm formation have used not only Arabidopsis but also S. tuberosum, as plant models. The ability of potato periderm to generate new phellem cells in only a few days in response to wounding, as compared to several years in cork oak, was recognized as a valuable attribute for suberin research (Pollard et al. 2008). Through the potato and Arabidopsis studies, a variety of enzymes were identified to play principal roles in suberin biosynthesis. Enzymes identified included fatty acid oxidases of the CYP86A sub-family, cytochrome P450 oxidases required for ω-hydroxy acid biosynthesis, StCYP86A33 (Beck 2010; Macnee et al. 2020; Woolfson et al. 2022), an acyl-activating enzyme of the LACS family, and acyltransferases of the GPAT family (Pollard et al. 2008). Several additional enzymes were later discovered involved in suberization, such as StKCS6 (3-ketoacyl-CoA-synthase), which alters the profile of very long-chain fatty acids and its derivatives, discovered when this enzyme was silenced in potato periderms (Serra et al. 2009a). The importance of ferulic acid in suberin biosynthesis was reinforced using Arabidopsis mutant lines with a reduction in phenylpropanoid (PP) synthesis (Andersen et al. 2021). The exogenous application of ferulic acid, one of the main aromatic monomers supplied by PP, conducted to new suberin deposition. Also, downregulation of StFHT (fatty alcohol hydroxycinnamoyl transferase) was involved in binding aliphatic compounds to ferulate, impairing the water barrier and producing tissue disorganization of potato periderm (Serra et al. 2010).
Silencing of StCYP86A33 gene revealed a 60% decrease in aliphatic suberin, a 60% reduction of glycerol esterified to suberin, and a reduction in 18:1 ω-hydroxy acids and ω,α-dicarboxylic acids in potato tuber periderm (Serra et al. 2009b; Lulai and Neubauer 2014) demonstrated that the genes StPAL-1 (phenylalanine ammonia-lyase 1), StTHT (tyramine hydroxycinnamolyl transferase), StFHT, StKCS6, StFAOH (fatty acid omega hydroxylation) and StGPAT5 participate in processing the closing layer and wound periderm development in wounded potato periderm (Lulai and Neubauer 2014). The potato TF, StNAC103, is involved in repression of suberin and wax deposition. Silencing this gene resulted in increased suberin and wax load (alkanes, ω-hydroxyacids, diacids, ferulic acid, and primary alcohols). Also, four genes involved in wax and suberin accumulation (KAR, FHT, CYP86A33, and WBC11) have been transcriptionally up-regulated in the periderm of StNAC103-RNAi transgenic lines of potato (Verdaguer et al. 2016). The FAR enzymes (AtFAR1, AtFAR2, and AtFAR5), responsible for the conversion of fatty acids to C18:0–C22:0 primary alcohols, were described in loss-of-function mutants/triple-far mutants in Arabidopsis, indicating an important role in the fatty alcohols formation (Domergue et al. 2010). In addition, some transcripts of genes involved in fatty-acid elongation during suberin biosynthesis, such as QsKCS, QsFAR, QsLACS, and QsGPAT, were also described as highly up-regulated in Q. suber (Teixeira et al. 2014; Lopes et al. 2019).
Recently, two sets of GELP/GDSL-type Esterase/Lipase Proteins complex (GDSL) were also described in Arabidopsis roots involved in suberin synthesis and degradation, regulating suberin plasticity in roots. The GELP22, GELP38, GELP49, GELP51, and GELP96 can be involved in aliphatic suberin assembly, while GELP12, GELP55, and GELP72 may mediate suberin degradation (Ursache et al. 2021; Serra and Geldner 2022). Upregulated GDSL in the suberized skins of tomato and apple with a potential role in suberin biosynthesis were also identified by Lashbrooke et al. (2016).
The knowledge of the function of these key enzymes will be important to understand the suberin role not only in biotic protection and stress tolerance, considering the climatic change context, but also in healing wounds after cork harvest or during the post-harvest period in cork oak.
Cork quality
As previously mentioned, cork tissue (phellem) is comprised of several compacted layers of suberized cells in cork oak. A uniform high-quality cork layer, known as amadia cork, is only obtained after around 40 years of tree growth, after the third harvesting, representing the high quality needed for producing wine stoppers, the most valuable use of cork (Graça and Pereira 2004; Pereira 2015). The amadia cork produced by traumatic phellogen, has the best characteristics for industrial transformation, as opposed to the first cork (virgin cork) known for its poor quality (Graça and Pereira 2004). Cork plank quality is based upon a combination of structure discontinuities, such as degree of porosity and a number of inclusions of schlerenchymatic cells, and thickness quality grades (Fig. 4) (Pereira 2007). Porosity corresponds to the presence of lenticels or lenticular channels, varying in number and dimensions, creating pores in cork from exterior to interior (Fig. 1) (Ghalem et al. 2016), and playing a role in gas exchange between the atmosphere and the internal plant tissues (Carrillo-López and Yahia 2019). Lenticels are biological structures originating from the activity of a particular secondary meristem, lenticular phellogen, filled with non-suberized tissue (Pereira 2007). Lenticels induce a protrusion in the epidermis and arise from beneath stomata or group of stomata between stoma. They are readily visible, corresponding to the fissures present in the cork (Groh et al. 2002). Cork with a low number of lenticels, having small diameter pores, is generally considered of high industrial quality. Lenticular channels cross cork layers radially (Rosner and Kartusch 2003). For this reason, bottle stoppers are cut out of cork planks at right angles to the surface so that the lenticels (pores) extend horizontally, rather than vertically, which would lead to leakage of the contents (Dickison 2000)
Cork planks with imperfections such as “nails” or inclusions of schlerenchymatic cells (i.e., inclusion in the cork tissue possessing areas of thick-walled lignified cells formed by death phellogen), insect galleries, and stains resulting from microbial disease, may present lower quality for the industry (Gonzalez-Adrados and Pereira 1996; Pereira 2007). Another factor in determining cork quality is its thickness. Thickness is based on cumulative cork growth and depends upon activity of the phellogen to create new layers of tissue annually. To achieve the quality characteristics required for the cork stopper industry, cork planks must have a standard thickness of at least 27 mm, an insignificant number of pores or other defects, and consistent color and texture (Silva et al. 2005; Pereira 2007; Inácio et al. 2017).
In addition to cork wine stoppers, there are other applications for which cork may be used, such as agglomerates, insulation boards, fashion and accessories (e.g., hats, shoes, and handbags), decorations, ornaments, etc. For these products, cork that is rejected by the stopper industry or, in general, poor quality cork can be used despite the defects (Graça and Pereira 2004; Silva et al. 2005; Pereira et al. 2008).
The production of high-quality cork for the industry has been restricted worldwide to cork oak. But, other species have the potential to produce bark with high cork content.
Other cork-rich bark species
Other oak species from the same section Cerris, Q. variabilis Blume and Q. cerris var. cerris Boiss, have the ability to produce a cork-rich bark. However, both species have a modest cork production and a lower cork quality, when compared to Q. suber.
Q. variabilis (Chinese cork oak) has a continuous periderm like Q. suber, but with a smaller ring width, that regenerates if removed (Ferreira et al. 2016b; Leite and Pereira 2017). It has been already commercially exploited for agglomerate material on a small scale in China. But due to its cork properties, higher density, compressive strength, and elasticity, the production of solid cork products, such as wine stoppers, is not appropriate (Miranda et al. 2013; Leite and Pereira 2017).
Q. cerris var. cerris (previously Q. cerris var. Pseudocerris), Turkey oak, has sequential periderms (3–4) with dead phloem tissue between them (Şen et al. 2011a). Therefore, the raw material is considered with inferior quality when compared to the cork from Q. suber. Due to scarcity of the cork imported from Portugal and Spain during World War II, the Q. cerris bark was used in Turkey (Kasapligil 1981). Unfortunately, from that date, few studies with this bark were performed (Şen et al. 2011a, b). Nowadays, the importance of Q. cerris counts on its major ecological role, and therefore the bark is not commercially exploited.
Other tree species with abundant suberification on the bark can be highlighted. Phellodendron amurense (Family Rutaceae), Amur cork tree, native from Eastern Asia has a large number of cork tissues in the outer bark that becomes fissured and corky with age (Azad et al. 2013). Besides its potential uses for industrial cork, the bark has been mainly used in traditional Chinese medicine (Azad et al. 2005). The phellogen is also very active for many years in Ulmus minor Mill or Ulmus campestre var. suberosa Moench, Field elm, being responsible for the production of its corky bark (Zajączkowska 2016). The bark of Acer campestre L. (Family Sapindaceae), English field maple, has also a corky star-shaped section on young branches and when the tree matures it turns furrowed (Mills 1996).
Genetic and epigenetic mechanisms linked to cork quality
Abiotic stress, such as drought and/or long-term exposure to high temperatures, reduces phellogen activity, affecting cork growth, and, consequently, cork quality (Mendes et al. 2016). However, recent studies indicate genetic and epigenetic factors play a significant role in defining cork quality (Ramos et al. 2013; Lopes et al. 2019).
Comparisons of transcriptional profiles of phellogen tissue yielding good and bad quality cork were evaluated. These studies took into consideration the principal traits defining cork quality, such as thickness and structural discontinuities including the degree of porosity and inclusion of woody cells. Results showed that these quality attributes were determined by different environmental stress-response pathways (Teixeira et al. 2014, 2018). Transcriptional profiles from high-quality cork showed suberin-associated genes were highly expressed. This demonstrated synthesis of lignin and suberin, being end-products of the phenylpropanoid pathway, is more activated in trees producing superior quality cork (Teixeira et al. 2018). The cytochrome P450 family 86 subfamily A polypeptide 1 (QsCYP86A1) orthologue, associated with biosynthesis of suberin monomers, was highly expressed in good-quality cork samples. This higher expression resulted in production of more suberized cork cells and, consequently, thicker cork layers in good-quality compared to poorer-quality cork trees (Teixeira et al. 2014). Conversely, higher accumulation of phenolic compounds and upregulation of candidate genes related to the structure of cell walls observed in bad-quality cork samples suggest that the flavonoid biosynthetic pathway is used instead of the suberin synthesis (Teixeira et al. 2018). In addition, knowing that drought and heat plays important role in the quality of cork, some heat shock protein such as HSP17.5-E, HSP17.6 C, HSP26.5, and HSP22.7 were found more expressed in bad-cork quality, and HSP70-15 more expressed in good-cork quality (Mendes et al. 2022).
In bad-quality cork, a lower level of global methylation was detected, with a trend for higher expression level of DNA methyltransferases (DNMTs) genes (Ramos et al. 2013). Also, QsDRM2 (Domain Rearranged Methyltransferase 2, responsible for de novo methylation) and QsMET2 (Methyltransferase 2) responsible for establishing and maintaining DNA methylation), presented the highest expression in derived cells from phellogen, which can explain due to its proliferation and differentiation activity.
The basic helix-loop-helix (bHLH), SPCH(SPEECHLESS), FAMA, and MUTE orthologues, essential genes for stomata formation (Pillitteri and Torii 2012), and the precursors for lenticular channel formation (Pereira 2007), were more expressed in bad-quality cork (Teixeira et al. 2014). Nearly all cork samples having a high pore-count per given area (a trait typifying bad-quality cork) exhibited an unmethylation state of two MSAP (Methylation Sensitive Amplification Polymorphism) sequences (Inácio et al. 2017). A positive relation between QsMET1 (Methyltransferase 1) and the number of pores in cork was found, suggesting the involvement of QsMET1 in the silencing pathway of suberin production genes, as lenticel filling cells have a low content of suberin (Inácio et al. 2018). However, QsMET2 and QsSUVH4 (histone-lysine N-methyltransferase) expression negatively correlated to pore length and roundness, respectively.
The methylation polymorphism was also associated with a low percentage of cork defect “nails” in good-quality cork (Inácio et al. 2017). QsDMAP1 (DNA methyltransferase 1 associated protein 1), a gene associated with DNA repair and cell control, was more expressed in cells from cork exhibiting quality defects. Its higher expression in poor-quality cork signifies a putative repair role (Ramos et al. 2013).
A comparison between different industrial qualities of cork (virgin vs. amadia cork) revealed a higher number of up-regulated genes associated with disease resistance, heat shock proteins, and hormone signaling in virgin cork, while in amadia cork certain transcripts associated with heavy metal homeostasis and abiotic stresses were observed or highlighted (Lopes et al. 2019). Differences in methylation profiles between virgin and amadia corks were also found (Inácio et al. 2017), hypothesizing that after traumatic chromatin remodeling during amadia differentiation, some memory can be imprinted in the newly formed phellogen, contributing to these differences in methylation patterns.
Conclusion
This review serves as a compilation of studies highlighting the importance of periderm function, an essential barrier between the vasculature and the atmosphere, focusing on cork. Cork oak is a unique woody species with particular traits concerning chemical composition and genetic activity of periderm, with an extraordinary active and long-living phellogen. Research on this secondary meristem, phellogen, has been somewhat overlooked compared with the vast literature available for vascular cambium in woody species. The lack of raw materials for the cork industry, the diversity of cork quality between individuals, together with the decline of cork oak woodlands are some of the key problems to overcome in near future.
Nowadays, important periderm-specific genomic resources are available for cork oak, increasing our knowledge of molecular mechanisms in periderm formation and differentiation in woody species, namely in oaks, and it will boost our understanding of biological processes behind cork quality. This also involves taking into consideration new efforts for gene discovery, functional genomics, and engineering biosynthesis of suberin, among others. Due also to the good secondary suberization, this species can be used as a good periderm model in woody species to study the formation of both original (first) and secondary (traumatic, after debarking) periderms (Lopes et al. 2019; Inácio et al. 2021). Despite the high level of knowledge acquired over the years with the cork oak, the long-life cycle of the slow-growth oak could be a bottleneck in the advancement in this area. The analysis and comparison with other species with cork-rich barks, like fast-growing Ulmus minor, may help to clarify many aspects of cork formation in the future. The causes of natural variability in cork quality are also an issue that deserves the attention of researchers, in which the early genomic prediction will help to manage the high-quality cork individuals. Advances in the development of genomic tools on phellem research and its integration with other omics analyses will also benefit forest industries to produce value-added products, in addition to cork quality and its properties.
References
Almeida T, Menéndez E, Capote T et al (2013a) Molecular characterization of Quercus suber MYB1, a transcription factor up-regulated in cork tissues. J Plant Physiol 170(2):172–178. https://doi.org/10.1016/j.jplph.2012.08.023
Almeida T, Pinto G, Correia B et al (2013b) QsMYB1 expression is modulated in response to heat and drought stresses and during plant recovery in Quercus suber. Plant Physiol Biochem 73:274–281. https://doi.org/10.1016/j.plaphy.2013.10.007
Andersen TG, Molina D, Kilian J et al (2021) Tissue-Autonomous Phenylpropanoid production is essential for establishment of Root barriers. Curr Biol 31:965–977e5. https://doi.org/10.1016/j.cub.2020.11.070
Aronson J, Pereira JS, Pausas JG (2009) Cork oak woodlands on the edge: ecology, adaptive management, and restoration. Society for Ecological Restoration International. Island Press, London, p 315
Arzee T, Arbel E, Cohen L (1977) Ontogeny of Periderm and Phellogen Activity in Ceratonia siliqua L. Bot Gaz 138:329–333
Azad MAK, Yokota S, Ishiguri F et al (2005) Histological studies of shoot regeneration system in hypocotyl-derived callus of Phellodendron amurense Rupr. J For Res 10(5):377–384. https://doi.org/10.1007/s10310-005-0148-9
Azad MAK, Yoshizawa N, Yokota S, Ishiguri F (2013) Somatic embryogenesis of Phellodendron amurense: a histomorphological study. In: Aslam J, Srivastava PS, Copyright MPS (eds) Somatic Embryogenesis and Genetic Transformation in plants. Narosa Publishing House, pp 121–143
Beck CB (2010) An Introduction to Plant Structure and Development: Plant Anatomy for the Twenty-First Century. An Introduction to Plant Structure and Development. https://doi.org/10.1017/cbo9780511844683
Beisson F, Li-Beisson Y, Pollard M (2012) Solving the puzzles of cutin and suberin polymer biosynthesis. Curr Opin Plant Biol 15:329–337. https://doi.org/10.1016/j.pbi.2012.03.003
Bernards MA (2002) Demystifying suberin. Canad.J Bot 80:227–240. https://doi.org/10.1139/b02-017
Boher P, Soler M, Sánchez A et al (2018) A comparative transcriptomic approach to understanding the formation of cork. Plant Mol Biol 96:103–118. https://doi.org/10.1007/s11103-017-0682-9
Camilo-Alves C, Dinis C, Vaz M et al (2020) Irrigation of Young Cork Oaks under Field Conditions—Testing the best water volume. Forests 11(1):88. https://doi.org/10.3390/f11010088
Campilho A, Nieminen K, Ragni L (2020) The development of the periderm: the final frontier between a plant and its environment. Curr Opin Plant Biol 53:10–14. https://doi.org/10.1016/j.pbi.2019.08.008
Capote T, Barbosa P, Usié A et al (2018) ChIP-Seq reveals that QsMYB1 directly targets genes involved in lignin and suberin biosynthesis pathways in cork oak (Quercus suber). BMC Plant Biol 18:198. https://doi.org/10.1186/s12870-018-1403-5
Carrillo-López A, Yahia EM (2019) Morphology and anatomy in: Yahia EM (ed): Postharvest physiology and biochemistry of fruits and vegetables. Woodhead Publishing, pp113-130. https://doi.org/10.1016/b978-0-12-813278-4.00006-3
Chapin FS, Schulze ED, Mooney HA (2003) The Ecology and Economics of Storage in plants. Annu Rev Ecol Syst 21:423–447. https://doi.org/10.1146/annurev.es.21.110190.002231
Coder KD (2019) Tree anatomy: periderm (Bark). Outreach Publication WSFNR-19-37
Conde E, García-Vallejo MC, Cadahía E (1999) Variability of suberin composition of reproduction cork from Quercus suber throughout industrial processing. Holzforschung 53:56–62. https://doi.org/10.1515/hf.1999.010
Cordeiro N, Belgacem MN, Silvestre AJD et al (1998) Cork suberin as a new source of chemicals.1. Isolation and chemical characterization of its composition. Int J Biol Macromol 22:71–80. https://doi.org/10.1016/s0141-8130(97)00090-1
Costa A, Pereira H, Oliveira A (2003) Variability of radial growth in cork oak adult trees under cork production. For Ecol Manage 175:239–246. https://doi.org/10.1016/S0378-1127(02)00145-7
Costa A, Catelanunes L, Spiecker H, Graça J (2015) Insights into the responsiveness of Cork Oak (Quercus suber L.) to bark harvesting. Econ Bot 69(2):171–184. https://doi.org/10.1007/s12231-015-9305-z
Costa R, Lourenço A, Oliveira V, Pereira H (2019) Chemical characterization of cork, phloem and wood from different Quercus suber provenances and trees. Heliyon 5:e02910. https://doi.org/10.1016/j.heliyon.2019.E02910
Crang R, Lyons-Sobaski S, Wise R (2018) Periderm. Plant Anatomy 553–575. https://doi.org/10.1007/978-3-319-77315-5_16
Crouvisier-Urion K, Chanut J, Lagorce A et al (2019) Four hundred years of cork imaging: new advances in the characterization of the cork structure. Sci Rep 9:1–10. https://doi.org/10.1038/s41598-019-55193-9
Dickison WC (2000) Integrative plant anatomy. Academic Press. https://doi.org/10.1016/b978-0-12-215170-5.X5000-6
Domergue F, Vishwanath SJ, Joubès J et al (2010) Three Arabidopsis fatty acyl-coenzyme A reductases, FAR1, FAR4, and FAR5, generate primary fatty alcohols associated with suberin deposition. Plant Physiol 153:1539–1554. https://doi.org/10.1104/pp.110.158238
Duque-Lazo J, Navarro-Cerrillo RM, Ruíz-Gómez FJ (2018) Assessment of the future stability of cork oak (Quercus suber L.) afforestation under climate change scenarios in Southwest Spain. For Ecol Manage 409:444–456. https://doi.org/10.1016/j.foreco.2017.11.042
Faustino A, Pires RC, Capote T et al (2022) Overexpression of QsMYB1 gene from Quercus suber induces suberin alteration in transgenic potato plants. In: III International Meeting of the Portuguese Society of Genetics. Évora, 27–28
Fernández-Piñán S, Boher P, Soler M et al (2021) Transcriptomic analysis of cork during seasonal growth highlights regulatory and developmental processes from phellogen to phellem formation. Sci Rep 11(1):12053. https://doi.org/10.1038/s41598-021-90938-5
Ferreira JPA, Miranda I, Gominho J, Pereira H (2016a) Chemical characterization of cork and phloem from Douglas fir outer bark. Holzforschung 70:475–483. https://doi.org/10.1515/hf-2015-0119
Ferreira J, Miranda I, Şen U, Pereira H (2016b) Chemical and cellular features of virgin and reproduction cork from Quercus variabilis. Ind Crops Prod 94:638–648. https://doi.org/10.1016/j.indcrop.2016.09.038
Fortes M, Rosa ME, Pereira H (2004) A cortiça. IST Press, Lisbon
Gandini A, Neto CP, Silvestre AJD (2006) Suberin: a promising renewable resource for novel macromolecular materials. Prog Polym Sci 31:878–892. https://doi.org/10.1016/j.progpolymsci.2006.07.004
Ghalem A, Barbosa I, Tarik Bouhraoua R, Costa A (2016) Comparing cork quality from Hafir-Zarieffet mountain forest (Tlemcen, Algeria) vs. tagus basin Montado (Benavente, Portugal). Cogent Biol 2(1):1236431. https://doi.org/10.1080/23312025.2016.1236431
Gil L (2014) Cork: a strategic material. Front Chem 2:16. https://doi.org/10.3389/fchem.2014.00016
Gonzalez-Adrados JR, Pereira H (1996) Classification of defects in cork planks using image analysis. Wood Sci Technol 30:207–215. https://doi.org/10.1007/bf00231634
Gou M, Hou G, Yang H et al (2017) The MYB107 transcription factor positively regulates suberin biosynthesis. Plant Physiol 173:1045–1058. https://doi.org/10.1104/pp.16.01614
Graça J (2015) Suberin: the biopolyester at the frontier of plants. Front Chem 3:62. https://doi.org/10.3389/fchem.2015.00062/bibtex
Graça J, Pereira H (1997) Cork suberin: a glyceryl based polyester. Holzforschung 51:225–234. https://doi.org/10.1515/hfsg.1997.51.3.225
Graça J, Pereira H (2000) Suberin structure in Potato Periderm: glycerol, Long-Chain Monomers, and glyceryl and feruloyl dimers. J Agric Food Chem 48:5476–5483. https://doi.org/10.1021/jf0006123
Graça J, Pereira H (2004) The periderm development in Quercus suber. IAWA J 25:325–335. https://doi.org/10.1163/22941932-90000369
Graça J, Santos S (2007) Suberin: a biopolyester of plants’ skin. Macromol Biosci 7:128–135. https://doi.org/10.1002/mabi.200600218
Groh B, Hübner C, Lendzian KJ (2002) Water and oxygen permeance of phellems isolated from trees: the role of waxes and lenticels. Planta 215:794–801. https://doi.org/10.1007/s00425-002-0811-8
Harman-Ware AE, Sparks S, Addison B, Kalluri UC (2021) Importance of suberin biopolymer in plant function, contributions to soil organic carbon and in the production of bio-derived energy and materials. Biotechnol Biofuels 14:75. https://doi.org/10.1186/S13068-021-01892-3
Heldt H-W, Piechulla B (2021) Plant biochemistry, 5th edn. Academic Press
Holloway PJ (1983) Some variations in the composition of suberin from the cork layers of higher plants. Phytochemistry 22:495–502. https://doi.org/10.1016/0031-9422(83)83033-7
Inácio V, Barros PM, Costa A et al (2017) Differential DNA Methylation Patterns Are Related to Phellogen Origin and Quality of Quercus suber Cork. PLoS One 12(1):e0169018. https://doi.org/10.1371/journal.pone.0169018
Inácio V, Martins MT, Graça J, Morais-Cecílio L (2018) Cork oak young and traumatic PeridermsShow PCD Typical Chromatin Patterns but Different Chromatin-Modifying GenesExpression. Front Plant Sci 9:1194. https://doi.org/10.3389/fpls.2018.01194
Inácio V, Lobato C, Graça J, Morais-Cecílio L (2021) Cork cells in cork oak periderms undergo programmed cell death and proanthocyanidin deposition. Tree Physiol 41(9):1701–1713. https://doi.org/10.1093/treephys/tpab031
Jin L, Cai Q, Huang W et al (2018) Potato native and wound periderms are differently affected by down-regulation of FHT, a suberin feruloyl transferase. Phytochemistry 147:30–48. https://doi.org/10.1016/j.phytochem.2017.12.011
Kasaplıgil B (1981) Past and present oaks of Turkey. Phytologia 49:95–146
Kolattukudy PE (1980) Biopolyester membranes of plants: cutin and suberin. Science 208:990–1000. https://doi.org/10.1126/science.208.4447.990
Kosma DK, Murmu J, Razeq FM et al (2014) AtMYB41 activates ectopic suberin synthesis and assembly in multiple plant species and cell types. Plant J 80:216–229. https://doi.org/10.1111/tpj.12624
Kumar A, Korpinen R, Möttönen V, Verkasalo E (2022) Suberin fatty acid hydrolysates from outer Birch Bark for hydrophobic coating on aspen wood surface. Polymers 14(4):832
Lashbrooke J, Cohen H, Levy-Samocha D et al (2016) MYB107 and MYB9 homologs regulate suberin deposition in angiosperms. Plant Cell 28:2097–2116. https://doi.org/10.1105/tpc.16.00490
Lavorel S, Canadell J, Rambal S, Terradas J (1998) Mediterranean Terrestrial Ecosystems: Research Priorities on Global Change Effects. Global Ecol Biogeogr Lett 7:157. https://doi.org/10.2307/2997371
Leal S, Nunes E, Pereira H (2008) Cork oak (Quercus suber L.) wood growth and vessel characteristics variations in relation to climate and cork harvesting. Eur J For Res 127(1):33–41. https://doi.org/10.1007/s10342-007-0180-8
Leal AR, Barros PM, Parizot B et al (2022a) Translational profile of developing phellem cells in Arabidopsis thaliana roots. Plant J 110:899–915. https://doi.org/10.1111/tpj.15691
Leal AR, Sapeta H, Beeckman T et al (2022b) Spatiotemporal development of suberized barriers in cork oak taproots. Tree Physiol 42:1269–1285. https://doi.org/10.1093/treephys/tpab176
Legay S, Guerriero G, Deleruelle A et al (2015) Apple russeting as seen through the RNA-seq lens: strong alterations in the exocarp cell wall. Plant Mol Biol 88:21–40. https://doi.org/10.1007/s11103-015-0303-4
Legay S, Guerriero G, André C et al (2016) MdMyb93 is a regulator of suberin deposition in russeted apple fruit skins. New Phytol 212:977–991. https://doi.org/10.1111/nph.14170
Leite C, Pereira H (2017) Cork-containing barks—a review. Front Mater 3:63. https://doi.org/10.3389/fmats.2016.00063/BIBTEX
Lev-Yadun S (2011) Bark. eLS John Wiley & Sons, Ltd. https://doi.org/10.1002/9780470015902.A0002078.pub2
Lopes ST, Sobral D, Costa B et al (2019) Phellem versus xylem: genome-wide transcriptomic analysis reveals novel regulators of cork formation in cork oak. Tree Physiol 40:129–141. https://doi.org/10.1093/treephys/tpz118
Louro G, Lonteiro ML, Constantino L, Rego FC (2014) The portuguese forest based chains: sector analyses. In: Reboredo F (ed) Forest context and policies in Portugal: present and future challenges. Springer, Cham, pp 39–67
Lu D, Wang T, Persson S et al (2014) Transcriptional control of ROS homeostasis by KUODA1 regulates cell expansion during leaf development. Nat Commun 5:3767. https://doi.org/10.1038/ncomms4767
Lulai EC, Neubauer JD (2014) Wound-induced suberization genes are differentially expressed, spatially and temporally, during closing layer and wound periderm formation. Postharvest Biol Technol 90:24–33. https://doi.org/10.1016/j.postharvbio.2013.11.010
Machado A, Pereira H, Teixeira RT (2013) Anatomy and development of the endodermis and phellem of Quercus suber L. roots. Microsc Microanal 19(3):525–534. https://doi.org/10.1017/S1431927613000287
Macnee NC, Rebstock R, Hallett IC et al (2020) A review of current knowledge about the formation of native peridermal exocarp in fruit. Funct Plant Biol 47:1019–1031. https://doi.org/10.1071/fp19135
Mahmood K, Zeisler-Diehl VV, Schreiber L et al (2019) Overexpression of ANAC046 promotes suberin biosynthesis in roots of Arabidopsis thaliana. Int J Mol Sci 20(24):6117. https://doi.org/10.3390/ijms20246117
Marques AV, Rencoret J, Gutiérrez A (2016) Ferulates and lignin structural composition in cork. Holzforschung 70:275–289. https://doi.org/10.1515/hf-2015-0014/pdf
Mendes MP, Ribeiro L, David TS, Costa A (2016) How dependent are cork oak (Quercus suber L.) woodlands on groundwater? A case study in southwestern Portugal. For Ecol Manage 378:122–130. https://doi.org/10.1016/j.foreco.2016.07.024
Mendes B, Usié A, Capote T et al (2022) Quercus suber transcriptome analyses: identification of genes and SNPs related to Cork Quality. Biol Life Sci Forum. https://doi.org/10.3390/iecps2021-11916
Miguel A, Milhinhos A, Novák O et al (2016) The SHORT-ROOT-like gene PtSHR2B is involved in Populus phellogen activity. J Exp Bot 67:1545–1555. https://doi.org/10.1093/jxb/erv547
Mills E (1996) An appreciation and natural history of the English Field maple (Acer Campestre L). Arboricultural J 20:405–410. https://doi.org/10.1080/03071375.1996.9747135
Miranda I, Gominho J, Pereira H (2013) Cellular structure and chemical composition of cork from the chinese cork oak (Quercus variabilis). J Wood Sci 59(1):1–9. https://doi.org/10.1007/S10086-012-1300-8
Moreira F, Duarte I, Catry F, Acácio V (2007) Cork extraction as a key factor determining post-fire cork oak survival in a mountain region of southern Portugal. For Ecol Manag 253(1–3):30–37. https://doi.org/10.1016/j.foreco.2007.07.001
Natividade JV (1950) Subericultura. Lisboa: Ministério da Economia. Direcção Geral dos Serviços Florestais e Aquícolas
Oliveira G, Costa A (2012) How resilient is Quercus suber L. to cork harvesting? A review and identification of knowledge gaps. For Ecol Manage 270:257–272. https://doi.org/10.1016/j.foreco.2012.01.025
Oliveira G, Martins-Loução MA, Correia O (2002) The relative importance of cork harvesting and climate for stem radial growth of Quercus suber L. Ann For Sci 59(4):439–443. https://doi.org/10.1051/forest:2002018
Pereira H (1988) Chemical composition and variability of cork from Quercus suber L. Wood Sci Technol 22:211–218. https://doi.org/10.1007/bf00386015
Pereira H (2007) Cork - Biology, production and uses. Elsevier, Amsterdam
Pereira H (2015) The rationale behind cork properties: a review of structure and chemistry. BioResources 10:1–23. https://doi.org/10.15376/biores.10.3pereira
Pereira H, Tomé M (2004) Cork Oak / non-wood products. Encyclopedia of Forest Sciences. Elsevier, pp 613–620
Pereira H, Graça J, Baptista C (1992) The Effect of Growth Rate on the structure and Compressive Properties of Cork. IAWA J 13:389–396. https://doi.org/10.1163/22941932-90001294
Pereira JS, Bugalho MN, Caldeira MDC (2008) From the Cork Oak to cork. APCOR - Portugese Cork Association
Pereira-Leal JB, Abreu IA, Alabaça CS et al (2014) A comprehensive assessment of the transcriptome of cork oak (Quercus suber) through EST sequencing. BMC Genomics 15:1–14. https://doi.org/10.1186/1471-2164-15-371
Pillitteri LJ, Torii KU (2012) Mechanisms of stomatal development. Annu Rev Plant Biol 63:591–614. https://doi.org/10.1146/annurev-arplant-042811-105451
Pires RC, Ferro A, Capote T et al (2022a) Laser microdissection of Woody and Suberized Plant Tissues for RNA-Seq analysis. Mol Biotechnol. https://doi.org/10.1007/s12033-022-00542-9
Pires RC, Capote T, Ferro A, Marum L (2022b) Transcriptome analysis in Cork Oak using laser microdissection and RNA-Seq. Bio Life Sci Forum 79. https://doi.org/10.3390/iecps2021-11914
Pollard M, Beisson F, Li Y, Ohlrogge JB (2008) Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci 13:236–246. https://doi.org/10.1016/j.tplants.2008.03.003
Ricardo CPP, Martins I, Francisco R et al (2011) Proteins associated with cork formation in Quercus suber L. stem tissues. J. Proteomics 74(8):1266–1278. https://doi.org/10.1016/j.jprot.2011.02.003
Rains MK, De Silva NDG, Molina I (2017) Reconstructing the suberin pathway in poplar by chemical and transcriptomic analysis of bark tissues. Tree Physiol 38:340–361. https://doi.org/10.1093/treephys/tpx060
Ramos M, Rocheta M, Carvalho L et al (2013) Expression of DNA methyltransferases is involved in Quercus suber cork quality. Tree Genet Genomes 9:1481–1492. https://doi.org/10.1007/s11295-013-0652-6
Ramos AM, Usié A, Barbosa P et al (2018) The draft genome sequence of cork oak. Sci Data 2018 5:180069. https://doi.org/10.1038/sdata.2018.69
Rosner S, Kartusch B (2003) Structural changes in primary lenticels of Norway spruce over the seasons. IAWA J 24:105–116. https://doi.org/10.1163/22941932-90000324
Sabba RP, Lulai EC (2002) Histological analysis of the maturation of native and Wound Periderm in Potato (Solanum tuberosum L.) Tuber. Ann Bot 90:1–10. https://doi.org/10.1093/aob/mcf147
Şen A, Quilhó T, Pereira H (2011) Bark anatomy of Quercus cerris L. var. cerris from Turkey. Turkish J Bot 35(1):45–55. https://doi.org/10.3906/bot-1002-33
Şen A, Quilhó T, Pereira H (2011b) The cellular structure of cork from Quercus cerris var. Cerris bark in a materials’ perspective. Ind Crops Prod 34(1):929–936. https://doi.org/10.1016/j.indcrop.2011.02.015
Serra O, Geldner N (2022) The making of suberin. New Phytol. https://doi.org/10.1111/nph.18202
Serra O, Soler M, Hohn C et al (2009a) Silencing of StKCS6 in potato periderm leads to reduced chain lengths of suberin and wax compounds and increased peridermal transpiration. J Exp Bot 60:697–707. https://doi.org/10.1093/jxb/ern314
Serra O, Soler M, Hohn C et al (2009b) CYP86A33-targeted gene silencing in potato tuber alters suberin composition, distorts suberin lamellae, and impairs the periderm’s water barrier function. Plant Physiol 149:1050–1060. https://doi.org/10.1104/pp.108.127183
Serra O, Hohn C, Franke R et al (2010) A feruloyl transferase involved in the biosynthesis of suberin and suberin-associated wax is required for maturation and sealing properties of potato periderm. Plant J 62:277–290. https://doi.org/10.1111/J.1365-313x.2010.04144.x
Serra O, Mähönen AP, Hetherington AJ, Ragni L (2022) The making of Plant Armor: the Periderm. Annu Rev Plant Biol 73:405–432. https://doi.org/10.1146/annurev-arplant-102720-031405
Shukla V, Han JP, Cléard F et al (2021) Suberin plasticity to developmental and exogenous cues is regulated by a set of MYB transcription factors. Proc Natl Acad Sci U S A 118(39):e2101730118. https://doi.org/10.1073/pnas.2101730118
Sillero L, Prado R, Andrés MA, Labidi J (2019) Characterisation of bark of six species from mixed Atlantic forest. Ind Crops Prod 137:276–284. https://doi.org/10.1016/j.indcrop.2019.05.033
Silva SP, Sabino MA, Fernandas EM et al (2005) Cork: Properties, capabilities and applications. Int Mater Rev 50:345–365. https://doi.org/10.1179/174328005x41168
Simonson WD, Allen HD, Parham E et al (2018) Modelling biodiversity trends in the montado (wood pasture) landscapes of the Alentejo, Portugal. Landsc Ecol 33:811–827. https://doi.org/10.1007/s10980-018-0627-y
Soler M, Serra O, Molinas M et al (2007) A genomic Approach to Suberin Biosynthesis and Cork differentiation. Plant Physiol 144:419–431. https://doi.org/10.1104/pp.106.094227
Soler M, Serra O, Molinas M et al (2008) Seasonal variation in transcript abundance in cork tissue analyzed by real time RT-PCR. Tree Physiol 28:743–751. https://doi.org/10.1093/treephys/28.5.743
Soler M, Serra O, Fluch S et al (2011) A potato skin SSH library yields new candidate genes for suberin biosynthesis and periderm formation. Planta 233:933–945. https://doi.org/10.1007/s00425-011-1350-Y
Soler M, Verdaguer R, Fernández-Piñán S et al (2020) Silencing against the conserved NAC domain of the potato StNAC103 reveals new NAC candidates to repress the suberin associated waxes in phellem. Plant Sci 291:110360. https://doi.org/10.1016/j.plantsci.2019.110360
Teixeira RT, Pereira H (2010) Suberized cell walls of cork from cork oak differ from other species. Microsc Microanal 16:569–575. https://doi.org/10.1017/s1431927610093839
Teixeira RT, Fortes AM, Pinheiro C, Pereira H (2014) Comparison of good- and bad-quality cork: application of high-throughput sequencing of phellogenic tissue. J Exp Bot 65:4887–4905. https://doi.org/10.1093/jxb/eru252
Teixeira RT, Fortes AM, Bai H et al (2018) Transcriptional profiling of cork oak phellogenic cells isolated by laser microdissection. Planta 247:317–338. https://doi.org/10.1007/s00425-017-2786-5
Ursache R, Teixeira CJV, Tendon VD et al (2021) GDSL-domain proteins have key roles in suberin polymerization and degradation. Nat Plants 7:353–364. https://doi.org/10.1038/s41477-021-00862-9
Verdaguer R, Soler M, Serra O et al (2016) Silencing of the potato StNAC103 gene enhances the accumulation of suberin polyester and associated wax in tuber skin. J Exp Bot 67:5415–5427. https://doi.org/10.1093/jxb/erw305
Vessella F, Parlante A, Schirone A et al (2010) Irrigation regime as a key factor to improve growth performance of Quercus suber L. Scand J For Res 25(8):68–74. https://doi.org/10.1080/02827581.2010.485819
Wacowska M (1985) Ontogenesis and structure of periderm in Acer negundo L. and x Fatshedera lizei Guillaum. Acta Soc Bot Pol 54:11–27
Wei X, Mao L, Wei X et al (2020) MYB41, MYB107, and MYC2 promote ABA-mediated primary fatty alcohol accumulation via activation of AchnFAR in wound suberization in kiwifruit. Hortic Res 7:1–10. https://doi.org/10.1038/s41438-020-0309-1
Woolfson KN, Haggitt ML, Zhang Y et al (2018) Differential induction of polar and non-polar metabolism during wound-induced suberization in potato (Solanum tuberosum L.) tubers. Plant J 93:931–942. https://doi.org/10.1111/tpj.13820
Woolfson KN, Esfandiari M, Bernards MA (2022) Suberin biosynthesis, Assembly, and Regulation. Plants (Basel) 11(4):555. https://doi.org/10.3390/plants11040555
Wunderling A, Ripper D, Barra-Jimenez A et al (2018) A molecular framework to study periderm formation in Arabidopsis. New Phytol 219:216–229. https://doi.org/10.1111/nph.15128
Xiao W, Molina D, Wunderling A et al (2020) Pluripotent pericycle cells trigger different growth outputs by integrating developmental cues into distinct Regulatory Modules. Curr Biol 30:4384–4398e5. https://doi.org/10.1016/j.cub.2020.08.053
Zajączkowska U (2016) Cork. In eLS, John Wiley & Sons, Ltd (Ed.). https://doi.org/10.1002/9780470015902.a0002080.pub2
Acknowledgements
Dr. Bruce Campbell (Western Regional Research Center, USDA-ARS, Albany CA, USA) is gratefully acknowledged for the English review of this manuscript.
Funding
Open access funding provided by FCT|FCCN (b-on). The present work was supported by Program Alentejo 2020 under the scope of Lentidev—A molecular approach to cork porosity (ALT20-03-0145-FEDER-000020), and through FCT under the projects UIDB/05183/2020 to Mediterranean Institute for Agriculture, Environment and Development (MED), and LA/P/0121/2020 to Global Change and Sustainability Institute (CHANGE). Authors also acknowledge FCT to Contrato – Programa to L. Marum (CEECINST/00131/2018), and the Ph.D. grant of A. Faustino (UI/BD/153511/2022).
Author information
Authors and Affiliations
Contributions
Conceptualization—LM; Investigation—AF and RCP; Resources—AF, RCP, and LM; Literature search and data analysis—AF, RCP. and LM; Writing—original draft preparation, AF, RCP and LM.; Writing review and editing—AF, RCP and LM; Funding acquisition, LM. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Communicated by G. Piovesan .
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Faustino, A., Pires, R.C. & Marum, L. Periderm differentiation: a cellular and molecular approach to cork oak. Trees 37, 627–639 (2023). https://doi.org/10.1007/s00468-023-02398-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-023-02398-1