Abstract
From construction and demolition of buildings, brick wastes accumulate in large quantities and are difficult to recycle. Re-using bricks as planting substrate could partly substitute gravel or other raw materials, and would reduce deposition of bricks in landfills. High water-holding capacity and a high specific surface of such substrates are beneficial for plant growth, while high pH could be a disadvantage. This study focuses on potential effects of brick-based substrates on survival, growth and functional traits of two urban trees (Acer platanoides, Tilia cordata). We compared the effects of brick quantity (5 vs. 30%), pre-treatment with phosphoric acid, nutrient-poor vs. -rich soil, and mycorrhiza inoculation upon saplings in two greenhouse experiments. There were no effects on survival, while a high brick ratio slightly reduced growth of A. platanoides and its branching in nutrient-rich soil, and tend to increase the root-to-shoot ratio in both species. The acid pre-treatment caused negative effects on relative growth rate of A. platanoides. Mycorrhiza inoculation had a tendency for a positive effect on growth in T. cordata, but only with 5% brick ratio. Overall, the brick-based substrates have no clear effect on the study species. Thus, bricks can be recommended as a neutral component within constructed Technosols, and can be used to modify grain size distribution without negative effects on survival, growth and performance, while further studies are needed on bricks with cement and gypsum contaminations.
Highlights
-
Brick rubble is a promising component of urban plant substrates.
-
Tree saplings of Acer platanoides and Tilia cordata have good survival and grow well on brick-based substrates.
-
Mycorrhiza inoculation has no effect on survival and growth or A. platanoides and T. cordata.
-
Acid pre-treatment of bricks has no effect on survival and growth of the tree saplings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Re-using demolition or production waste as component of novel plant substrates was advocated by several studies, that focused on brick and concrete rubble, coal dust, paper ash or sewage sludge (Du et al. 2020; Liu et al. 2019; Molineux et al. 2015; Naeth and Wilkinson 2014). Three billion tons of demolition waste was produced in 2012 across 40 countries, and these materials are frequently dumped in landfills with limited capacities (Akhtar and Sarmah 2018). For example, in Europe, landfill restrictions should be further strengthened to foster a circular economy (European Union 2018). Thus, re-using waste materials as a component of novel plant substrates has a high potential to spare landfills, to use less soil and save costs (Deeb et al. 2020; Walsh et al. 2018). Such ‘constructed Technosols’ are specific mixtures of wastes containing organic and/or mineral components, to achieve particular functions (Séré et al. 2008). They are cost-efficient (Walsh et al. 2018), though the main challenge is a suitable balance of contrasting components (Rokia et al. 2014).
Brick rubble is a promising material for constructed Technosols. It makes up a large proportion of construction waste (Akhtar and Sarmah 2018), and it would make more sense to re-use bricks for novel substrates than to use them as bulk material for backfill, as is currently the case. Bricks can store plant-available nutrients at their surfaces like P, K and Mg, and their water-holding capacity is high (Nehls et al. 2013). Fine roots can enter up to 7% of the brick pores, enabling the root hairs to exploit most of the brick water (Nehls et al. 2013). This is due to the small pores that can effectively conduct the suction exerted by the root throughout the brick (Nehls et al. 2013). However, the high pH (9–10) of bricks must be taken into account during substrate development (Molineux et al. 2009; Rokia et al. 2014), while a combination with organic material can (but not necessarily need to) reduce the pH to 7–8 (cf. Hitchmough et al. 2001; Molineux et al. 2009). Similarly, a treatment of bricks with phosphoric acid might reduce the pH and improve survival and soil fertility, but this still needs to be tested. Bricks are stable but easy to crush with machines and can therefore be used to reach a certain grain size distribution. However, because of their friability mainly due to frost, they should not be considered as a ‘structural material’ against soil compaction (Rokia et al. 2014), but rather as ‘growing material’ when mixed with organic matter (Yilmaz et al. 2018). Growing material is put directly around root balls and must have a higher water infiltration and water-holding capacity compared to structural material, which in turn should prevent soil compaction (Yilmaz et al. 2018).
Urban tree planting is a potential application for brick-augmented Technosols. Such substrates play a crucial role in urban green infrastructure, since they are relevant for plant growth and runoff infiltration (Deeb et al. 2020). Urban trees usually suffer high mortality due to soil compaction (Bartens et al. 2009), adverse water availability (Rhoades and Stipes 1999), nutrient deficiency and pollutants (Jim 1998). On the one hand, good maintenance programs should be planned in the first years after planting (Koeser et al. 2014), and on the other hand, constructed Technosols must be designed to prevent soil compaction and to increase water storage (Deeb et al. 2020). Brick-augmented substrates could satisfy these needs because they combine a relatively high bearing capacity (Bretzel et al. 2020) with a high water-holding capacity (Rokia et al. 2014). Even though the bearing capacity is high, Rokia et al. (2014) raise concerns to the sustainability because of the friability of bricks mainly due to frost. We suppose that bricks can reduce for a while compaction effects of trampling but not of heavy vehicles. Another possibility to reduce urban tree mortality is mycorrhiza inoculation. Mycorrhiza changes the rhizosphere's root morphology and functioning (Kothari et al. 1990), thus potentially mitigating the natural and anthropogenic stressors that cause nutrient shortage of plants (Entry et al. 2002).
Bricks were already used for tree experiments with Acer platanoides and Tilia cordata with brick proportions of 58 wt%, and 85 or 100 vol% (Bretzel et al. 2020; Cannavo et al. 2018). Bretzel et al. (2020) showed that bricks increase shoot and root length. However, these studies did not test the effects of brick-augmented substrates combined with mycorrhiza inoculation and acid pre-treatment. Furthermore, previous research focused on soil characteristics and less on functional plant traits.
Our study aims to test the effects of the brick-augmented substrate on survival, growth and functional traits of tree saplings. The study species were A. platanoides and T. cordata, i.e., typical urban trees with high drought and frost tolerance (Roloff et al. 2009). A. platanoides is a mid-successional, whereas T. cordata is a late-successional species, and they differ in two crucial traits, i.e., adults’ minimum light demand and the leaf area index (LAI) (Leuschner and Meier 2018). For T. cordata, the adults’ minimum light demand is lower and the LAI is higher than for A. platanoides (Leuschner and Meier 2018). Furthermore, they differ in their mycorrhiza type: A. platanoides has mainly an arbuscular mycorrhiza, while T. cordata has both arbuscular mycorrhiza and ectomycorrhiza (Wang and Qiu 2006).
We used relative growth rate (RGR), above- and belowground functional traits to investigate the effect of brick-augmented substrates on the growth of urban trees. The selected traits are affected by different site conditions like water availability or soil fertility (Chave et al. 2009; Weemstra et al. 2016; Wright et al. 2004). The root economic spectrum for trees is not yet consolidated (Weemstra et al. 2016), but both root architectural traits like branching intensity or allocation, such as root mass fraction, correlate with the widely accepted plant economic spectrum (Kramer-Walter et al. 2016; Kramer-Walter and Laughlin 2017; Liese et al. 2017). Though, specific root length (SRL) explains a substantial part of the variation in the multiple trait space of tree species, but is independent of specific leaf area (SLA) and the plant economic spectrum (Kramer-Walter et al. 2016).
To answer the following questions, we set up two factorial experiments with substrates of different brick quantities and soil types, a mycorrhiza treatment and an acid pre-treatment.
-
1.
How strong do substrates with an increased brick ratio affect mortality, growth and functional plant traits of trees?
-
2.
How much reduces a pre-treatment of bricks with phosphoric acid the brick addition effects on plants?
-
3.
Has mycorrhiza inoculation of brick-augmented substrate a positive effect on plant growth?
Materials and methods
Species, substrates, and treatments
Two experiments with two tree species were done planting them in substrates with different ratios of crushed bricks and mycorrhiza addition. The two selected species were deciduous broad-leaved trees, i.e., Acer platanoides (Sapindaceae; wfo-0000514884) and Tilia cordata (Malvaceae; wfo-0000457451, World Flora Online 2022). They were chosen because they are common urban trees and are considered suitable for future climate conditions with increasing droughts and frost events, and for high pH values of brick-based substrates (Eaton et al. 2021; Pasta et al. 2021; Roloff et al. 2009). A commercial nursery [WGS84 (lat/lon): 48.40003, 11.39534] supplied bare-rooted saplings (50–80 cm height, 2y transplanted), that were planted into containers with a volume of 12 l (diameter 33 cm; height 24 cm).
The used brick rubble was the crushed production waste of the brickyard Leipfinger-Bader with a grain size of 4–16 mm. Smaller grain size fractions are re-used within the production process, and larger fractions should be avoided due to the size of our experimental unit (container of 12 l). We used a maximum brick ratio of 30 vol%, since German regulations do not allow the coarse fraction (>4 mm) to exceed 49 wt% (Forschungsgesellschaft Landschaftsentwicklung Landschaftsbau 2010). The brick rubble was not contaminated by cement or gypsum; it had a pH 8.1 and a total pore volume 38%.
The brick rubble was added to two base substrates with a proportion of 5 or 30 vol% which were produced for the experiments by the Umwelt Wurzer company: the nutrient-rich substrate had 3 vol% of compost (0–15 mm) from their own composting facility which processes organic waste and green cutting, 27 vol% topsoil, 10 vol% sand, and 30 or 55 vol% gravel, while the nutrient-poor substrate had 10 vol% topsoils, 10 vol% sand, and 50 or 75vol% gravel. The nutrient-rich substrate fulfills the requirements of the grain size distribution for urban trees in Germany (See Appendix Figs. 5, 6; Forschungsgesellschaft Landschaftsentwicklung Landschaftsbau 2010).
The substrates with 5 vol% bricks had a pH 7.6–7.7, and the ones with 30 vol%, 7.7–8.1; the nutrient-rich substrate, which contained compost, had lower pH values than the poor substrate (Table 1). The organic matter proportion in the nutrient-rich substrate was 2.3–2.7 wt%, and 0.7 wt% in the nutrient-poor substrate. A pre-treatment of bricks with phosphoric acid was tested. For that, 0.3 mol kg−1 H3PO4 was used and added with the bricks to a concrete mixer which run for 4 min. Acid-treated bricks increased phosphate [plus 13–37 mg 100 g−1 (min–max)].
For the mycorrhiza treatment, we used A. platanoides ectomycorrhizal fungi with 0.03 l of ectomycorrhizal inoculant per container of the fungi species Cortinarius traganus and Scleroderma citrinum. The dosage for fungal inoculation in T. cordata was 0.015 l of ectomycorrhizal inoculant per container and 0.015 l endomycorrhizal inoculant of the species Glomus spec. (GEFA Produkte Fabritz GmbH, Krefeld).
Experimental design
Both experiments were conducted in a greenhouse at the Greenhouse Laboratory Centre Dürnast of the Technical University of Munich (WGS 84 (lat/lon): 48.40526, 11.68909). The investigation started at the end of March 2019 and ran for 67 weeks, over two seasons, until the beginning of July 2020. We stopped the experiment when the pots were completely rooted. In winter, the greenhouse was heated to avoid temperatures <5 ℃, and in summer, the ventilation operated at >10 ℃. No artificial light was used, all trees were watered equally but irregularly on demand (irrigation water pH 7.8) and never fertilized. We divided the experiment into two factorial experiments with a fully randomized block design and five replicates per experiment. Therefore, five blocks were formed, and each block was within one row (Fig. 1). Each row had an own pipe system which watered the pots. We re-randomized the trees within each row in June and December 2019 to avoid edge effects.
The fully randomized block design for the first experiment with four treatments (= 16 treatment combinations) and five replicates (= blocks) and in total 80 pots with tree saplings. The experiments were situated in a greenhouse and the pots of each block were connected with a pipe system to the row’s water tap
Both experiments consisted of both species, two substrates (nutrient-rich, nutrient-poor), and two brick ratios (5 vol%, 30 vol%). The specific treatment of the first experiment was the mycorrhiza fungi inoculation or not (with or without), with all bricks treated with phosphoric acid. For the second experiment, all trees were inoculated with mycorrhiza, but tested acid pre-treated bricks for the brick ratio of 30 vol%. In total, the first experiment had 80 trees and the second experiment 60 trees (Table 2). We controlled spider mites (Tetranychidae) with insecticide sprays, beneficial insects (Phytoseiulus persimilis, Amblyseius californicus) and adhesive strips.
Measurements and data analysis
We analyzed the substrates for grain size distribution, pH, nutrients and organic ratio (Table 1, See Appendix Table 4, Appendix Figs. 5, 6). Soil texture was classified according to the “Bodenkundliche Kartieranleitung” (Bundesanstalt für Geowissenschaften und Rohstoffe 2005). The pH was measured in CaCl2 (Verband deutscher landwirtschaftlicher Untersuchungs- und Forschungsanstalten 1991). Organic matter was derived from the ignition loss (550 ℃) (DIN 2002). Plant available phosphorus and potassium were measured in a calcium acetate–lactate extract and magnesium in CaCl2 extract (fraction <2 mm).
After planting, survival was recorded, and the initial height and stem diameter were measured. The height was taken from the soil’s surface to the highest apical bud and the diameter 2 cm above the soil with an electronic digital caliper. The final height and stem diameter were measured before harvesting. We calculated the relative growth rate (\({\mathrm{RGR}}_{{d}^{2}h}\)) as:
where, \({d}_{f}\) and \({d}_{i}\) are the final and initial diameter, \({h}_{f}\) and \({h}_{i}\) the final and initial height, and \(t\) denotes time (in days) between the initial and final growth measurements (Baltzer and Thomas 2007). We harvested above- and belowground biomass and dried it at 65 ℃ for three days and afterward we weighed it immediately. Before drying and weighing the roots, the roots were thoroughly cleaned from soil particles. Afterwards, leaf, stem, and root mass fractions were calculated by dividing one organ by its total biomass. The root-to-shoot ratio was calculated by dividing root mass by summed leaf and stem mass.
Leaves’ and roots’ functional traits were measured according to standard protocols (Pérez-Harguindeguy et al. 2013). We collected three leaf samples, including petioles, per tree, and stored them in a fridge for <7 days. Leaf area was measured with a flat-bed scanner and ImageJ (Version 1.53c; Schneider et al. 2012). Subsequently, leaves were dried at 65 ℃ for 3 days and then weighed. Specific leaf area (SLA) was calculated as leaf area divided by dry leaf mass.
Each tree's fine root samples had a diameter of <2 mm and were stored in a freezer. We washed and separated the samples into absorptive fine roots (1st–3rd order) and transport fine roots (>4th order) (McCormack et al. 2015). The absorptive fine roots’ length and volume were measured with a flat-bed scanner and the software WinRHIZO 2019 (Régent Instruments Inc., Quebec City, Canada; resolution: 800 dpi). All fine root samples were dried at 65 ℃ for three days and then weighed. We calculated specific root length (SRL) and root tissue density for absorptive fine roots by dividing root length by dry root mass and dividing dry root mass by root volume, respectively. The absorptive–transport fine root ratio was calculated by dividing the 1st–3rd order root mass by the 4th and higher-order root mass. The root branching intensity was calculated as the number of root tips divided by absorptive fine root length.
Statistical analyses
We log-transformed the data values if they were right-skewed distributed. For the first experiment, linear mixed-effects models were calculated with the random effect ‘block’ (= row) and the maximum likelihood method. For the second experiment, linear models were used if the random effect caused ‘singularity’ and explained no variance. If p < 0.05, we call it 'statistically clear' (Dushoff et al. 2019), and calculated post hoc tests with the Tukey-HSD method of each species separately. The uncertainties of the effects were expressed as standard error (SE). All analyses were performed in R (Version 4.0.2; R Core Team 2020), with the packages ‘lme4’ for linear mixed-effects models (Bates et al. 2015), ‘MuMIn’ for Pseudo-R2 values (Barton 2019), ‘DHARMa’ for model evaluation (Hartig 2020), ‘emmeans’ for calculating post hoc tests (Lenth 2020) and ‘ggeffects’ (function ‘ggemmeans’) to extract the graphs' coefficients (Breheny and Burchett 2017; Lüdecke 2018).
Results
The effect of increased brick addition
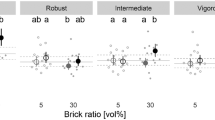
All plants survived well, irrespective of soil treatments. The biggest effects on plant traits due to increased brick ratio were for Acer platanoides in rich soils but the effects were not statistically clear. The relative growth rate (\({\mathrm{RGR}}_{{d}^{2}h}\)) was 27 ± 14% (mean ± SE) lower with 30% bricks than with 5% (t1,89.3 = 2.0, padj = 0.21), the stem mass fraction was 15 ± 7% lower (t1,88.6 = − 2.1, padj = 0.17), the root-to-shoot ratio was 18 ± 12% higher (t1,88.6 = 1.7, padj = 0.35) and the branching intensity was 17 ± 10% lower (t1,88.5 = − 1.5, padj = 0.44) (Fig. 2). For Tilia cordata, only in nutrient-poor soil, the root-to-shoot ratio had a bigger difference of + 17 ± 11% with brick addition (t1,88.3 = 1.6, padj = 0.38; Fig. 2).
Effects of brick ratio (5 vs. 30 vol%) and soil fertility on ten functional traits of Acer platanoides and Tilia cordata saplings. Data of experiment 1: all bricks treated with phosphoric acid. Shown are the estimated marginal means and the corresponding confidence intervals of 95%; n.s. = not statistically significant (p > 0.05). Open and filled circles represent brick ratios of 5 and 30 vol%, respectively
The effects of acid pre-treatment of bricks and mycorrhiza inoculation
The differences due to acid pre-treatment of bricks were also generally low or not statistically clear (Table 3). For A. platanoides, the RGR decreased with the acid pre-treatment (− 21 ± 8%; t1,64.4 = − 2.0, padj = 0.11; Fig. 3, Appendix Fig. 7). For T. cordata, the ratio of absorptive to transport fine roots increased by 94 ± 44% (t1,64.8 = 2.9, padj = 1.3e − 02). For SLA, the acid pre-treatment of bricks had only a small effect (max. + 12 ± 5%; t1,68.8 = 1.9, padj = 0.39). There was no statistically clear interaction of mycorrhiza with any plant trait (Table 2). Since there was not the expected effect by an increased brick ratio, mycorrhiza inoculation could not mitigate such an effect. There was a tendency for a positive effect on RGR but only for T. cordata on substrates with a brick ratio of 5% (+ 36 ± 15%, t1,89.3 = 2.4, padj = 8.7e − 02; Fig. 4, Appendix Fig. 8).
Effect of brick ratio (5 vs. 30 vol%) on the Acer platanoides and Tilia cordata saplings’ relative growth rate (\({\mathrm{RGR}}_{{d}^{2}h}\)) with acid-treated bricks (filled symbols). The RGR for A. platanoides decreased for the acid treatment by 21 ± 8% (mean ± SE). Data of experiment 2: all substrates were inoculated with mycorrhiza; n.s. = not statistically significant (p > 0.05). The estimated marginal means and the corresponding confidence intervals of 95% are shown
Effect of brick ratio (5 vs 30 vol%) and mycorrhiza inoculation on the Acer platanoides and Tilia cordata saplings’ relative growth rate (\({\mathrm{RGR}}_{{d}^{2}h}\)). The RGR of T. cordata increased by 36 ± 15% (mean ± SE). The estimated marginal means and the corresponding confidence intervals of 95% are shown. Data of experiment 1: all bricks treated with phosphoric acid; Control = no inoculation, n.s. = not statistically significant (p > 0.05)
Discussion
The greenhouse experiment with brick-augmented substrates showed no effects on sapling survival and growth, and only small effects on the functional traits of the species Acer platanoides and Tilia cordata. For instance, slight effects were observed for A. platanoides: brick addition reduced the relative growth rate (\({\mathrm{RGR}}_{{d}^{2}h}\)) in nutrient-rich soils and increased the root-to-shoot ratio. All in all, there seems to be no ecologically significant effect on both species’ functional traits, neither by bricks nor by acid pre-treatment of bricks. Besides, the mycorrhiza inoculation did not significantly affect the functional traits of trees after almost two growth periods.
Nehls et al. (2013) stated that bricks could be a valuable source of certain nutrients (K, Mg, Ca, S), but only in poor soils. In our experiment, an increased brick proportion increased K and Mg concentrations in both nutrient-poor and -rich substrates. Nevertheless, we could not detect an effect by an increased brick proportion on tree growth in any of the tested soil types. Similar to our study, Bretzel et al. (2020) analyzed brick-augmented substrates with T. cordata for 2 years and neither found a brick effect on trunk diameter increment nor on the shoot length when using 85 vol% bricks (6–30 mm fraction) mixed with 15 vol% compost. Though, in their study, root dry weight and total root length increased, which is consistent with the tendency of increased root-to-shoot ratio found in our study. The higher allocation to roots suggests a lower nutrient availability in brick-augmented substrates (Poorter et al. 2012).
Root tissue density and branching intensity were measured, which should change depending on the growing conditions, as they are considered part of the plant’s economic spectrum (Kramer-Walter et al. 2016; Liese et al. 2017). Only for A. platanoides in rich soils, the branching intensity tended to decrease with the addition of higher amounts of bricks to the substrate. Lower branching intensity suggests better growing conditions and fostering an acquisitive resource economic strategy. This effect is not reflected for the SLA, an essential trait of the leaf economic spectrum (Wright et al. 2004). Similarly, Kramer-Walter and Laughlin (2017) found for their four investigated species only a tendency for a decrease of branching intensity with soil fertility and no effect to the SLA. Moreover, the SRL, which is considered a vital root trait (Kramer-Walter et al. 2016), showed no significant tendency due to the bricks’ effects. Especially for pre-treatment with phosphoric acid, we expected a higher RGR since bricks alone do not affect plants’ phosphorus availability (Nehls et al. 2013). Although the substrates with acid-treated bricks had a higher P availability than substrates without acid-treated bricks (+ 108% for nutrient-poor substrates, and + 185% for nutrient-rich substrates), they did not affect RGR or functional plant traits. Furthermore, the acid treatment of the bricks was not recognizable in a change of the pH value of the whole substrate (0% nutrient-poor, and − 3% nutrient-rich).
Overall, the effect by an increased brick addition seems to be slight. This could be due to the used coarse fraction (4–16 mm), which has a lower specific surface than a smaller fraction (<2 mm), and a longer diffusion time, which leads to a lower impact on nutrient supply (Nehls et al. 2013). This suggestion is supported by Bretzel et al. (2020), who found a positive effect on mean shoot length by the brick fraction 0–30 mm but not by the fraction 6–30 mm compared to standard soil. There seems to be no nutritional deficiency by brick-augmented substrates on trees, as we could show with the analysis of functional plant traits and Bretzel et al. (2020) with the chlorophyll index analysis, which was not affected by bricks. In our experiment, the higher amount of organic matter did not mitigate any tendencies for effects by increased brick proportion. In contrast, Bretzel et al. (2020) showed that the addition of compost improved growth when a brick substrate with the fraction of 6–30 mm was used. Though, they substituted the compost at the expense of bricks, which improved in combination the growing conditions, but cannot disentangle the brick and the compost effect, as we did.
In our experiment, substrates with a higher brick proportion had a reduced gravel proportion, while other substrate components like topsoil or compost remained unchanged. This should have fostered growing conditions by increasing water availability with bricks compared to gravel. Bricks, in fact, have favorable infiltration rates due to their high porosity (Bretzel et al. 2020; Yilmaz et al. 2018), and the resulting high water-holding capacity should make bricks a promising addition for constructed Technosols (Rokia et al. 2014; Séré et al. 2008). This should be especially true when comparing bricks with crushed concrete, which has 43 wt% less water content (Rokia et al. 2014). Nevertheless, even though in our experiment the increased brick proportion should have increased water availability, the RGR and functional plant traits were not significantly affected. This means that the additional water which was stored in the 30% bricks was not sufficient to affect tree growth, although modern, highly porous bricks were used. Brick age has to be considered in substrate development, since bricks produced in former decades have a lower porosity.
Finally, in our experiment, the mycorrhiza inoculation did not modify the effect of increased brick addition. There was a tendency for a positive effect on T. cordata’s RGR when the substrate contained only 5% bricks, but the effect diminished with a higher brick ratio. Similarly, Fini et al. (2011) did not detect any significant mycorrhiza inoculation effect on T. cordata biomass or root-to-shoot ratio. Wiseman and Wells (2009) neither find any mycorrhiza inoculation effect on Acer species’ aboveground growth but found an effect on root length. Nevertheless, Fini et al. (2011) found higher physiological activity in inoculated trees. The result does not necessarily mean that mycorrhiza did not improve growth, because it could be that the trees were not infected, since successful infection of plants by commercial mycorrhizal inoculate is unreliable (Salomon et al. 2022).
Conclusion
We conclude that T. cordata and A. platanoides, which are tolerant to high pH values, are not significantly affected by brick-augmented substrates with a brick ratio of ≤30 vol%. Furthermore, according to our results, it is neither necessary nor helpful to conduct bricks pre-treatment with acid or inoculate mycorrhiza to the substrate to mitigate an effect by an increased brick proportion. Since bricks can be easily crushed, they are helpful to modify physical characteristics of substrates like the grain size distributions to meet legal requirements. Since there were almost no effects on functional plant traits, our results confirm the substrate analyses of other studies, which suggested no large effect of an increased brick proportion on substrates (Nehls et al. 2013; Rokia et al. 2014). Further studies should investigate brick-based substrates over a longer period in combination with larger pots.
Nevertheless, our results suggest that the main advantage is not an enhancement in plant growth, but in re-using waste material and modifying the physical characteristics of substrates. For this reason, higher amounts of bricks should be used in substrates. Deeb et al. (2020) stated that one of the most critical limits is the “social refusal” of re-used waste. Thus, future research should focus on non-clean waste bricks with mortar and plaster attachments and re-used brick rubble from old buildings with a lower porosity than bricks produced nowadays.
Author contribution statement
MB and JK made the experimental design, and all authors designed the substrates. MB set up the experiment, conducted the data sampling, performed the statistical analyses, and wrote a first draft of the manuscript. JK, MK and VH substantially contributed to later versions.
Data availability
Bauer M, Krause M, Heizinger V & Kollmann J (2022) Data and code for Bauer et al. (2023) Trees (v1.0.0) [Data set]. Zenodo. https://doi.org/10.5281/zenodo.6390128.
References
Akhtar A, Sarmah AK (2018) Construction and demolition waste generation and properties of recycled aggregate concrete: a global perspective. J Clean Prod 186:262–281. https://doi.org/10.1016/j.jclepro.2018.03.085
Baltzer JL, Thomas SC (2007) Determinants of whole-plant light requirements in Bornean rain forest tree saplings. J Ecol 95:1208–1221. https://doi.org/10.1111/j.1365-2745.2007.01286.x
Bartens J, Day SD, Harris JR, Wynn TM, Dove JE (2009) Transpiration and root development of urban trees in structural soil stormwater reservoirs. Environ Manag 44:646–657. https://doi.org/10.1007/s00267-009-9366-9. Accessed 15 Mar 2022
Barton K (2019) MuMIn: multi-model inference. R package version 1.43.15. www.CRAN.R-project.org/package=MuMIn
Bates D, Mächler M, Bolker BM, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Breheny P, Burchett W (2017) Visualization of regression models using visreg. R J 9:56–71. https://doi.org/10.32614/RJ-2017-046
Bretzel F, Vannucchi F, Pini R, Scatena M, Marradi A, Cinelli F (2020) Use of coarse substrate to increase the rate of water infiltration and the bearing capacity in tree plantings. Ecol Eng 148:105798. https://doi.org/10.1016/j.ecoleng.2020.105798
Bundesanstalt für Geowissenschaften und Rohstoffe (ed) (2005) Bodenkundliche Kartieranleitung, 5th edn. Schweizerbart, Stuttgart
Cannavo P, Guénon R, Galopin G, Vidal-Beaudet L (2018) Technosols made with various urban wastes showed contrasted performance for tree development during a 3-year experiment. Environ Earth Sci 77:650. https://doi.org/10.1007/s12665-018-7848-x
Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE (2009) Towards a worldwide wood economics spectrum. Ecol Lett 12:351–366. https://doi.org/10.1111/j.1461-0248.2009.01285.x
Deeb M, Groffman PM, Blouin M, Egendorf SP, Vergnes A, Vasenev V, Cao DL, Walsh D, Morin T, Séré G (2020) Using constructed soils for green infrastructure–challenges and limitations. Soil 6:413–434. https://doi.org/10.5194/soil-6-413-2020
Deutsches Institut für Normung (DIN) (2002) DIN 18128:2002–12. Berlin. https://dx.doi.org/10.31030/9287613https://doi.org/10.31030/9287613
Du T, Wang D, Bai Y, Zhang Z (2020) Optimizing the formulation of coal gangue planting substrate using wastes: the sustainability of coal mine ecological restoration. Ecol Eng 143:105669. https://doi.org/10.1016/j.ecoleng.2019.105669
Dushoff J, Kain MP, Bolker BM (2019) I can see clearly now: Reinterpreting statistical significance. Methods Ecol Evol 8:12. https://doi.org/10.1111/2041-210X.13159
Eaton E, Caudullo G, de Rigo D (2021) Tilia cordata, Tilia platyphyllos and other limes in Europe: distribution habitat, usage and threats. In: San-Miguel-Ayanz J, de Rigo D, Caudullo G, Houston Durrant T, Mauri A (eds) European atlas of forest tree species. Publication Office of the European Union, Luxembourg, p e010ec5+
European Union (EU) (2018) Directive (EU) 2018/850 of the European parliament and the council of 30 May 2018 amending directive 1999/31/EC on the landfill of waste: Directive (EU) 2018/850, vol 61. http://data.europa.eu/eli/dir/2018/850/oj
Entry JA, Rygiewicz PT, Watrud LS, Donnelly PK (2002) Influence of adverse soil conditions on the formation and function of arbuscular mycorrhizas. Adv Environ Res 7:123–138. https://doi.org/10.1016/S1093-0191(01)00109-5
Fini A, Frangi P, Amoroso G, Piatti R, Faoro M, Bellasio C, Ferrini F (2011) Effect of controlled inoculation with specific mycorrhizal fungi from the urban environment on growth and physiology of containerized shade tree species growing under different water regimes. Mycorrhiza 21:703–719. https://doi.org/10.1007/s00572-011-0370-6
Forschungsgesellschaft Landschaftsentwicklung Landschaftsbau (FLL) (2010) Empfehlungen für Baumpflanzungen. Teil 2. Standortvorbereitungen für Neupflanzungen; Pflanzgruben und Wurzelraumerweiterung, Bauweisen und Substrate. Bonn.
Hartig F (2020) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.2.7. www.CRAN.R-project.org/package=DHARMa. Accessed 15 Mar 2022
Hitchmough J, Kendle T, Paraskevopoulou AT (2001) Seedling emergence, survival and initial growth of forbs and grasses native to Britain and central/southern Europe in low productivity urban “waste” substrates. Urban Ecosyst 5:285–308. https://doi.org/10.1023/A:1025643929335
Jim CY (1998) Urban soil characteristics and limitations for landscape planting in Hong Kong. Landsc Urban Plan 40:235–249. https://doi.org/10.1016/S0169-2046(97)00117-5
Koeser AK, Gilman EF, Paz M, Harchick C (2014) Factors influencing urban tree planting program growth and survival in Florida, United States. Urban For Urban Green 13:655–661. https://doi.org/10.1016/j.ufug.2014.06.005
Kothari SK, Marschner H, George E (1990) Effect of VA mycorrhizal fungi and rhizosphere microorganisms on root and shoot morphology, growth and water relations in maize. New Phytol 116:303–311. https://doi.org/10.1111/j.1469-8137.1990.tb04718.x
Kramer-Walter KR, Laughlin DC (2017) Root nutrient concentration and biomass allocation are more plastic than morphological traits in response to nutrient limitation. Plant Soil 416:539–550. https://doi.org/10.1007/s11104-017-3234-9
Kramer-Walter KR, Bellingham PJ, Millar TR, Smissen RD, Richardson SJ, Laughlin DC (2016) Root traits are multidimensional: specific root length is independent from root tissue density and the plant economic spectrum. J Ecol 104:1299–1310. https://doi.org/10.1111/1365-2745.12562
Lenth R (2020) Emmeans: estimated marginal means, aka least-sqares means. R package version 1.4.4. www.CRAN.R-project.org/package=emmeans. Accessed 15 Mar 2022
Leuschner C, Meier IC (2018) The ecology of Central European tree species: trait spectra, functional trade-offs, and ecological classification of adult trees. Perspect Plant Ecol Evol Syst 33:89–103. https://doi.org/10.1016/j.ppees.2018.05.003
Liese R, Alings K, Meier IC (2017) Root branching is a leading root trait of the plant economics spectrum in temperate trees. Front Plant Sci 8:315. https://doi.org/10.3389/fpls.2017.00315
Liu X, Liu L, Leng P, Hu Z (2019) Feasible and effective reuse of municipal sludge for vegetation restoration: physiochemical characteristics and microbial diversity. Sci Rep 9:879. https://doi.org/10.1038/s41598-018-37338-4
Lüdecke D (2018) Ggeffects: tidy data frames of marginal effects from regression models. J Open Source Softw 3:1–5. https://doi.org/10.21105/joss.00772
McCormack ML, Dickie IA, Eissenstat DM, Fahey TJ, Fernandez CW, Guo D, Helmisaari H-S, Hobbie EA, Iversen CM, Jackson RB, Leppälammi-Kujansuu J, Norby RJ, Phillips RP, Pregitzer KS, Pritchard SG, Rewald B, Zadworny M (2015) Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol 207:505–518. https://doi.org/10.1111/nph.13363
Molineux CJ, Fentiman CH, Gange AC (2009) Characterising alternative recycled waste materials for use as green roof growing media in the U.K. Ecol Eng 35:1507–1513. https://doi.org/10.1016/j.ecoleng.2009.06.010
Molineux CJ, Gange AC, Connop SP, Newport DJ (2015) Using recycled aggregates in green roof substrates for plant diversity. Ecol Eng 82:596–604. https://doi.org/10.1016/j.ecoleng.2015.05.036
Naeth MA, Wilkinson SR (2014) Establishment of restoration trajectories for upland tundra communities on diamond mine wastes in the Canadian Arctic. Restor Ecol 22:534–543. https://doi.org/10.1111/rec.12106
Nehls T, Rokia S, Mekiffer B, Schwartz C, Wessolek G (2013) Contribution of bricks to urban soil properties. J Soils Sediments 13:575–584. https://doi.org/10.1007/s11368-012-0559-0
Pasta S, de Rigo D, Caudullo G (2021) Acer pseudoplatanus in Europe: distribution, habitat, usage and threats. In: San-Miguel-Ayanz J, de Rigo D, Caudullo G, Houston Durrant T, Mauri A (eds) European atlas of forest tree species. Publication Office of the European Union, Luxembourg, p e01665a+
Pérez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Bret-Harte MS, Cornwell WK, Craine JM, Gurvich DE, Urcelay C, Veneklaas EJ, Reich PB, Poorter L, Wright IJ, Ray P, Enrico L, Pausas JG, de Vos AC, Buchmann N, Funes G, Quétier F, Hodgson JG, Thompson K, Morgan HD, ter Steege H, Sack L, Blonder B, Poschlod P, Vaieretti MV, Conti G, Staver AC, Aquino S, Cornelissen JHC (2013) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61:167. https://doi.org/10.1071/BT12225
Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L (2012) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol 193:30–50. https://doi.org/10.1111/j.1469-8137.2011.03952.x
R Core Team (2020) R: a language and environment for statistical computing. www.R-project.org. Accessed 15 Mar 2022
Rhoades RW, Stipes RJ (1999) Growth of trees on the Virginia Tech Campu in response to various factors. J Arboric 25:211–217
Rokia S, Séré G, Schwartz C, Deeb M, Fournier F, Nehls T, Damas O, Vidal-Beaudet L (2014) Modelling agronomic properties of Technosols constructed with urban wastes. Waste Manag 34:2155–2162. https://doi.org/10.1016/j.wasman.2013.12.016
Roloff A, Korn S, Gillner S (2009) The climate-species-satrix to select tree species for urban habitats considering climate change. Urban For Urban Green 8:295–308. https://doi.org/10.1016/j.ufug.2009.08.002
Salomon MJ, Demarmels R, Watts-Williams SJ, McLaughlin MJ, Kafle A, Ketelsen C, Soupir A, Bücking H, Cavagnaro TR, van der Heijden M (2022) Global evaluation of commercial arbuscular mycorrhizal inoculants under greenhouse and field conditions. Appl Soil Ecol 169:104225. https://doi.org/10.1016/j.apsoil.2021.104225
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Séré G, Schwartz C, Ouvrard S, Sauvage C, Renat J-C, Morel JL (2008) Soil construction: a step for ecological reclamation of derelict lands. J Soils Sediments 8:130–136. https://doi.org/10.1065/jss2008.03.277
Verband deutscher landwirtschaftlicher Untersuchungs- und Forschungsanstalten (1991) Das VDLUFA Methodenhandbuch: Band I die Untersuchung von Böden. VDLUFA-Schriftenreihe, vol 68
Walsh D, Glass K, Morris S, Zhang H, McRae I, Anderson N, Alfieri A, Egendorf SP, Holberton S, Owrang S, Cheng Z (2018) Sediment exchange to mitigate pollutant exposure in urban soil. J Environ Manage 214:354–361. https://doi.org/10.1016/j.jenvman.2018.03.013
Wang B, Qiu Y-L (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363. https://doi.org/10.1007/s00572-005-0033-6
Weemstra M, Mommer L, Visser EJW, van Ruijven J, Kuyper TW, Mohren GMJ, Sterck FJ (2016) Towards a multidimensional root trait framework: a tree root review. New Phytol 211:1159–1169. https://doi.org/10.1111/nph.14003
Wiseman PE, Wells CE (2009) Arbuscular mycorrhizal inoculation affects root development of Acer and Magnolia species. J Environ Hortic 27:70–79. https://doi.org/10.24266/0738-2898-27.2.70
World Flora Online (2022) World Flora Online. http://www.worldfloraonline.org/. Accessed 16 Mar 2022
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas M-L, Niinemets Ü, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827. https://doi.org/10.1038/nature02403
Yilmaz D, Cannavo P, Séré G, Vidal-Beaudet L, Legret M, Damas O, Peyneau P-E (2018) Physical properties of structural soils containing waste materials to achieve urban greening. J Soils Sediments 18:442–455. https://doi.org/10.1007/s11368-016-1524-0
Acknowledgements
We thank Kai Steinmetz and his team of the Greenhouse Laboratory Centre Dürnast for technical help. We are thankful for the help for experimental setup and measuring by Holger Paetsch. We are grateful to the company Umwelt Wurzer for their extensive support of the entire project and especially for the supply of the six substrates. The project was funded by the Federal Ministry for Economic Affairs and Energy within the ZIM program (Zentrales Innovationsprogramm Mittelstand; ZF4025032SA8).
Funding
Open Access funding enabled and organized by Projekt DEAL. The project was funded by Bundesministerium für Wirtschaft und Energie (ZF4025032SA8).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
I declare that the authors have no competing interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Table 4, Figs. 5, 6, 7, 8.
The grain size distributions of the substrates used for experiment 1. All substrates were with acid-treated bricks. Four substrates are shown with varying soil fertility (rich vs. poor) and brick proportion (5 vs. 30%). Limits are shown for the substrates of urban tree substrates in Germany (Forschungsgesellschaft Landschaftsentwicklung Landschaftsbau 2010)
The grain size distributions of the substrates used for experiment 2 on effects of mycorrhiza-inoculated bricks on Acer platanoides and Tilia cordata saplings. All six substrates are shown with varying soil fertility (rich vs. poor), brick proportion (5 vs. 30%), and acid treatment of bricks (control vs. acid). Limits are shown for the substrates of urban tree substrates in Germany (Forschungsgesellschaft Landschaftsentwicklung Landschaftsbau 2010)
Effect of brick ratio (5 vs 30 vol%) on relative growth rate (\({\mathrm{RGR}}_{{d}^{2}h}\)) of Acer platanoides and Tilia cordata saplings with acid-treated bricks (filled symbols). The RGR for A. platanoides decreased for the acid treatment by 21 ± 8% (mean ± SE). The ratio of absorptive:transport fine roots of T. cordata increased by 94 ± 44%. Data is taken of experiment 2: all substrates inoculated with mycorrhiza; n.s. = not statistically significant (p > 0.05). Shown are the estimated marginal means and the corresponding confidence intervals 95%
Effects of brick ratio (5 vs 30 vol%) and mycorrhiza inoculation on ten functional traits of Acer platanoides and Tilia cordata saplings. Data from experiment 1: all bricks with phosporic acid. Shown are the estimated marginal means and the corresponding confidence intervals 95%; n.s. = not statistically significant (p > 0.05)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bauer, M., Krause, M., Heizinger, V. et al. Increased brick ratio in urban substrates has a marginal effect on tree saplings. Trees 37, 875–889 (2023). https://doi.org/10.1007/s00468-023-02391-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-023-02391-8