Abstract
Key message
This paper describes the micropropagation of Douglas-fir via somatic embryogenesis using media and methods available in the public domain.
Abstract
Douglas-fir is a conifer species with high and increasing interest for forest industries in both New Zealand and France. Delivery of the best trees to the forest from breeding programmes is currently constrained by an inability to effectively and reliably multiply selections through vegetative propagation. Somatic embryogenesis coupled with cryopreservation is essential biotechnology tool in conifers for scaling up variety design and production. The aim of this work was therefore to develop protocols for the initiation and proliferation of Douglas-fir embryogenic cell lines based on the modifications of previously published techniques and media available in the public domain, especially successful methods developed for P. radiata at Scion. Three years of initiation experiments have resulted in a simple and efficient protocol. Disinfection of whole cones (instead of seeds) was sufficient to prevent contamination. Immature zygotic embryos were excised from megagametophytes and placed onto a modified Litvay medium (Plant Cell Rep 4(6):325–328, 1985***). At optimal development stages of zygotic embryos, the average initiation percentage of embryogenic tissue was 69.3% when our most effective protocol was used. Proliferation of initiated cell lines on a Glitz formulation was challenging, with a high percentage of cell lines composed of a mixture of both embryonal masses and callus. 2,4-D at a lower concentration reduced the number of such mixed lines. Repeated liquid suspension and subculture of the embryogenic parts of the tissue was an efficient means to increase the number of embryogenic cell lines with sustained proliferation. Maltose added to the proliferation medium in place of sucrose improved fresh mass gain and consistently increased early somatic embryo patterning and growth. A sample of proliferating cell lines was successfully cryopreserved, thawed and somatic embryos were matured and germinated from these lines. The method may be of practical interest and provide new opportunities to realize increased genetic gain in this species through the clonal-assisted deployment of the best genetic material in both New Zealand and France.
Similar content being viewed by others
Introduction
Douglas-fir (Pseudotsuga menziesii [Mirb.] Franco) is the most important species in Pacific Northwest forests of the US and Canada, where it is native. In New Zealand, Douglas-fir accounts for approximately 6% (over 107,900 ha) of the sustainably managed exotic plantation forests, second only to Pinus radiata (MPI website, 2013, http://www.mpi.govt.nz). Douglas-fir performs better than radiata pine on some high-altitude, snow-prone sites, especially in Otago and Southland, and its timber attracts premium prices due to its superior stiffness and the natural durability of its early forming heartwood (Shelbourne et al. 2007).
Douglas-fir US provenance/progeny collections were initiated in New Zealand in the 1950s and ranged from California to Washington (Shelbourne et al. 2007; Dungey et al. 2012; Low et al. 2012). While commercial seed has been available for some time, genetically improved seed with substantial gains is now available via both open, and to a lesser extent, control-pollinated programmes (http://www.proseed.co.nz, http://www.duskyforestseed.co.nz). Douglas-fir produces abundant seed crops, however, these are at irregular intervals, from 2 to 10 years apart (http://www.proseed.co.nz). It is therefore unlikely that significant quantities of controlled-pollinated seed will be available to supply New Zealand’s seed requirement for this species for a few years to come.
In France, Douglas-fir is currently the most important conifer species after Pinus pinaster for plantation forestry, accounting for approximately 3% (over 401,000 ha) of the productive forests, with a mean of 7.2 million seedlings produced by commercial forest nurseries and planted per year (2009–2015 data, http://agriculture.gouv.fr/statistiques-annuelles-sur-les-ventes-de-graines-et-plants-forestiers). As a species with rapid growth and very attractive wood quality, Douglas-fir breeding has received significant support in Europe since the 1970s to develop adapted varieties (reviewed in Bastien et al. 2013).
A core breeding population was established in 1985 and 1986 by FCBA (formerly AFOCEL) and INRA from 998 progeny of previously tested US provenances (IUFRO tests) ranging from Washington to Oregon. Phenotypic and/or genotypic selection in these families resulted in the planting, between 1978 and 1991, of eight first-generation clonal seed orchards. The entire annual seed crop of Douglas-fir varieties currently originates from these eight orchards, providing significant genetic gains (e.g. + 20 to 35% in volume). Market needs are currently fulfilled but seed shortage is expected as the reforestation programmes increase (plantations are gradually going to reach maturity) and productivity of first-generation seed orchards will concomitantly remain stable or even decrease (http://www.france-douglas.com).
Climate change is thought to be partly responsible for erratic seed production of Douglas-fir orchards in recent years. As a result, the French genetic breeding programme has received renewed and significant support from the government to secure Douglas-fir wood supply together with maintaining good wood quality in the context of climate and socio-economic changes. New seed orchards are expected to be established by 2020 (Michaud et al. 2014) based on additional selection criteria, such as wood quality and resilience to environmental changes estimated from clonal tests (grafted clones).
Operational deployment of the best genetic material to the forests in both New Zealand and France could greatly benefit from the development of an efficient vegetative propagation system. This is the best way to ensure the capture of both additive and non-additive genetic variances. Ideally, this would be through tissue culture and rooted cutting technologies. As Douglas-fir matures rapidly under stool bed management, as well as often forming plagiotropic shoots, having valuable crosses cryopreserved would also ensure continuity of supply with juvenile propagules.
Somatic embryogenesis is the primary technology facilitating the delivery of all conifer biotechnology services and products, including rejuvenation of stock, cryopreservation of genetic resources, transgenic trees and field-proven clonal planting stock (Park et al. 2006; reviewed in; Lelu-Walter et al. 2013; Klimaszewska et al. 2015). The combination of somatic embryogenesis with genetic transformation technology may prove to be very useful for Douglas-fir as far as creating sexual sterility is concerned. Sterility is not only an important prerequisite for the field release of genetically modified organisms, effectively reducing the risk of unwanted transgene flow from forest plantations (Porth and El-Kassaby 2014), but may also be useful for this species which is well known for the spread of wilding plants on some sites if not carefully managed (Ledgard et al. 2005). Genetic transformation of Douglas-fir embryogenic tissue has already proven to be feasible (Gupta et al. 1988, 1989; Jiang 1991).
Methods for somatic embryogenesis of Douglas-fir have been under development for several decades (mainly in the US), with the first report from Durzan and Gupta in 1987 reporting initiation rates of up to 20–25% at the best collection date, obtained from immature zygotic embryos (ZEs) at the precotyledonary stage. Similar results (17–23% initiation rates) were achieved by Hong et al. (1992). More successful work (40–57% initiation rates) followed, incorporating complex media supplements, dissection methods and ZE health evaluation (Pullman et al. 2005, 2009a, b). In most cases, methodologies and media from somatic embryogenesis initiation to somatic embryo (SE) germination were patented (Pullman and Gupta 1994; Gupta 1996; Gupta and Pullman 1996; Gupta et al. 2004; Kong et al. 2011; Timmis et al. 2011) protecting the use of these for commercial application and available only for research purposes. Recent progress with improving somatic embryogenesis initiation and early cell line proliferation in P. radiata that resulted in average initiation rates of 70% of the 19 open and 20 control-pollinated families tested were very encouraging (Hargreaves et al. 2009, 2011). Because of the success of these new protocols, we wanted to test their success in other commercially important species such as Douglas-fir.
The aim of the work reported here was therefore to test and develop protocols based on the results with P. radiata, but adapted to Douglas-fir. We also aimed to develop somatic embryogenesis protocols that employed a simplified ZE excision technique and media available in the public domain.
Materials and methods
Plant material
Immature (green) cones were collected during the southern hemisphere summer (November–January) from mature trees of Douglas-fir from three sites throughout New Zealand. In the summer of 2008–2009, there were four collections (21-Nov-08, 6-Dec-08, 18-Dec-08, and 6-Jan-09) of one to three cones from each of five unrelated open-pollinated (OP) families established in the Scion Long Mile archive (38.1378°S, 176.2514°E) in Rotorua. There were two collections in the summer of 2011–2012 (21-Dec-11, and 30-Dec-11) of one or two cones from each of five unrelated OP families from trees at a commercial seed orchard (Proseed) located at Amberley in North Canterbury (43.1556°S, 172.7306°E). A last collection was done during the summer of 2012–2013 (16-Jan-13) of three to six cones from five unrelated OP families growing at Ernslaw One’s “Dusky” forest seed in Otago (45.943°S, 169.2610°E).
Cones were stored in loosely sealed plastic bags at 4 °C while explant excisions were carried out over a 1–2 week period. Green cones were broken apart and seeds were removed still attached to the cone bracts, these were then disinfected in hydrogen peroxide for 10 min (30%, 100 volume in strength, analytical reagent grade, diluted at 10 ml/100 ml of sterile water, plus surfactant) followed by three rinses in sterile water. From 2011 to 2012 onwards, a simplified disinfection procedure was adopted, where whole cones were submerged in 70% ethanol for approximately 30 min, cones were then opened and seeds were removed and briefly dipped in 70% ethanol prior to ZE extraction.
Following disinfection, intact megagametophytes (containing ZEs) were aseptically removed from the seed coats. For some treatments, ZEs were dissected (including associated suspensor tissue) from the megagametophyte under a stereo microscope. Excised ZEs or intact megagametophytes were placed onto the test media and Petri dishes were sealed with food grade plastic cling film (Gladwrap™). In the case of excised ZEs, no contact with the megagametophyte remained. Explants were incubated at 24 °C in the dark. Four weeks later, Petri dishes were opened in the laminar flow cabinet so that any build-up of ethylene was allowed to escape, and then the dishes were resealed and returned to the growth incubators for a maximum period of 9 weeks. During this incubation period, ZEs were assessed for embryogenic tissue formation.
Seeds (up to 10 per tree for most experiments and all seed for experiment 3) were sampled at each collection time and assessed for ZE development using an eight-stage scale. This scale was similar to that used by Owens and Blake (1985), but with two earlier stages (our stages 3–8 were equivalent to stages 1–6 of Owens and Blake 1985). At our stage 1, pro-embryos had formed and stage 2 showed early development of the pro-embryos. Stages 3–8 were “bullet” stages (bullet-shaped) embryos of increasing development up to the precotyledonary stage (stage 6). Stage 7 had a clearly developed epicotyl meristem and emerging cotyledons (early cotyledonary stage) and a clear whorl of cotyledons (cotyledonary stage) was present at stage 8 (stage 6 in Owens and Blake 1985).
Media for SE initiation and/or proliferation of embryogenic lines
Three media were selected for the initiation and/or proliferation experiments (Table 1). Briefly, the media were (a) a modified Litvay et al. (1985) formulation (Glitz) that has been very successful for both somatic embryogenesis initiation and embryogenic tissue proliferation of P. radiata (Hargreaves et al. 2009, 2011); (b) a patented formulation developed especially for Douglas-fir (BM1) by Gupta et al. (2004); (c) a medium developed for P. taeda and two other Pinus species (Becwar et al. 1987) based on modifications to a Murashige and Skoog (1962) formulation (MSG). The exact formulation of the various media tested for each sampling season (variations of Glitz, BM1 and MSG) are detailed below in the individual experiments.
Media were prepared and pH was adjusted to 5.7 prior to autoclaving (121 °C, 0.12 MPa, 20 min). Amino acids were filter-sterilised but not pH-adjusted, and were added to the autoclaved media. The media were then dispensed into Petri dishes, 90 mm diameter × 25 mm depth (LabServ®, ThermoFisher Scientific, Australia).
Initiation experiments
Experiment 1(2008/2009): initiation medium effect (Glitz vs BM)
Two initiation media were tested; Glitz and BM1 (Table 1). The number of explants per treatment ranged from 3 to 36 depending on the number of seeds available (where possible 6 explants were cultured per Petri dish). All cultures initiated on Glitz or BM1 were proliferated on Glitz with a 7–10 day subculture period.
Experiment 2 (2011/2012): initiation media comparison (Glitz vs MSG) and explant types (intact megagametophytes vs excised zygotic embryos)
Initiation media consisted of two formulations (MSG and Glitz, Table 1), each supplemented with either 1 (MSG, Glitz) or 0.5 (MSGB, GlitzB) mg L−1 2,4-D. Two explant types were used for each of the four initiation media (giving eight treatments in total for each OP family): intact megagametophytes-containing ZEs (“M” treatments), and excised ZEs from megagametophytes (“ZE” treatments). There were 6–18 explants placed on each of the treatments (6 explants per Petri dish) at each collection date for each of the five families.
Four weeks later, all responding explants were transferred to fresh initiation media of the same composition (Glitz, GlitzB, MSG, and MSGB).
Experiment 3 (2012/2013): culture of excised ZEs on GlitzB
In January 2013, an initiation test was performed using excised ZEs on GlitzB medium (six explants per Petri dish) for confirmation of the success of this medium for the initiation of Douglas-fir embryogenic cell lines.
Following initiation, vigorously proliferating tissue was suspended in liquid Glitz medium containing no plant growth regulators (Glitz0) at a density of 1 g of tissue in 5 ml of liquid medium in a sterile 25 ml McCartney universal glass vial. Suspended tissue was then dispensed using a pipette to fresh GlitzB semisolid medium (1 ml per plate). For cell lines which contained a mix of embryogenic tissue and calli (hard, yellow and nodular), the embryogenic portion of the tissue was selected for suspension, avoiding the calli as much as possible; this selection and resuspension was done repeatedly to remove all calli. Excess liquid medium was allowed to evaporate in the laminar flow for approximately 2–3 h before Petri dishes were wrapped. Any proliferating embryogenic tissue identified later during the experiment (up to 9 weeks) was treated in the same way. All other explants (slow growing lines and non-responsive explants) were transferred to fresh initiation medium (GlitzB).
Confirmed proliferating cell lines were suspended in liquid Glitz0 containing maltose instead of sucrose (GlitzM0) and transferred to Glitz medium (Table 1) with 30 g L−1 maltose (instead of sucrose) as a carbohydrate source (GlitzM) and subcultured every 7–10 days.
The number of replications per treatment varied in initiation experiments. This was due to the availability of cones on sampled trees in the seed orchards and the numbers of full seeds present in these cones.
Proliferation experiment: effect of carbohydrate source, tissue preparation method and cell density for proliferation of Douglas-fir embryogenic lines on Glitz medium
Four healthy Douglas-fir embryogenic cell lines were used to optimize proliferation protocols. Two Glitz media containing either 30 g L−1 sucrose (Glitz, Table 1) or 30 g L−1 maltose (GlitzM) were tested using two cell densities (100 or 200 mg per plate) and two tissue preparation methods (see below).
Tissue preparation method 1 consisted of placing 1 g of proliferating embryogenic tissue into a sterile 25-ml McCartney universal vial and adding 5 ml of liquid media without growth regulators (Glitz0 for lines maintained on medium containing sucrose, and GlitzM0 for lines maintained on medium containing maltose). The vial was shaken to disperse the cells and then the cells were vigorously and repeatedly drawn into and out of the pipette tip (5 ml natural Gilson, Scientific specialties Inc., CA, USA) until flowing freely. The amount of tissue required (100 or 200 mg inoculum corresponding to 0.5 or 1 ml, respectively) was then dispersed onto the surface of the semisolid media (Glitz or GlitzM). Petri dishes were left in the laminar flow to allow excess liquid to evaporate before being wrapped with food grade plastic cling film (Gladwrap™).
For tissue preparation method 2, 5 ml of liquid medium was placed into a sterile 25-ml McCartney bottle, the amount of embryogenic tissue required (either 100 or 200 mg) was collected and weighed directly into the liquid media (Glitz0 or GlitzM0). The vial was gently shaken side to side (5–10 times) to disperse the cell aggregates. All liquid, cells and cell aggregates were then drawn into a cut pipette tip and gently dispersed onto the surface of the solid media. Excess liquid media was immediately and carefully removed using a pipette. Petri dishes were covered with a lid and wrapped immediately with plastic cling film.
Four cell lines, all initiated on Glitz, were tested (29-2, 30-4, 110-3, and 132-6), each with five replications. Embryogenic tissue fresh masses (FM) were weighed at the time of subculture (T0, 100 or 200 mg) and after growth for 13 days (T13) to calculate the relative fresh biomass gain (%) computed as (FMT13 − FMT0)/FMT0 × 100. At the same time, acetocarmine staining (Gupta and Durzan 1987) of each of the treatments for each cell line was carried out to view actively dividing cells. Approximately 150 mg of tissue was placed in a well of a 52-well Nunc plate, and approximately 1 ml of acetocarmine (0.75% carmine, 45% acetic acid, 54.25% distilled water, Schneider CAROLINA™) was added and left for 3–5 min. The tissue was then removed from the stain using tweezers, 10–15 mg were placed onto a microscope slide, a drop of 50% glycerol was added and the cells were squashed with a cover slip.
Cryopreservation of proliferating embryogenic lines
A total of 71 proliferating embryogenic cell lines from the three initiation experiments were cryopreserved using protocols based on those developed for P. radiata (Hargreaves et al. 2002). Briefly, vigorously growing embryogenic tissue was removed from a proliferation medium they were cultured on 6–7 days after the last subculture and suspended in liquid Glitz0 or GlitzM0 medium containing 0.4 M sorbitol. Tissue was suspended at a ratio of 1 g FM to 2.5 ml of liquid Glitz0 or GlitzM0 + 0.4 M sorbitol. Suspended tissue (ca. 3.5 ml) was incubated in 25-ml Pyrex flasks on a shaker (60 rpm) at room temperature for 18–24 h in the dark. Following this pre-culture, an equal volume of cooled (0–1 °C) Glitz0 or GlitzM0 + 0.4 M sorbitol medium containing 20% (v/v) DMSO (dimethylsulfoxide) was added to the cell suspensions to give a final concentration of 10% (v/v) DMSO. Aliquots of 1 ml of suspended tissue were then transferred to 2 ml cryovials (Nunc) and kept on ice until being transferred to a programmable freezer (Model 7452 Series CryoMed Controlled Rate Freezer, Thermo Scientific). The programme used was based on Find et al. (1993), with a cooling rate of 0.5 °C min−1 to − 17.5 °C where it was held for 10 min, then 0.5 °C min−1 to − 40 °C. Following slow cooling, vials were transferred directly to liquid nitrogen. Tissue was recovered from liquid nitrogen using a nurse culture method as described previously for radiata pine (Hargreaves et al. 2002).
Maturation and germination of somatic embryos
For maturation experiments, samples of 100 mg of vigorously proliferating embryogenic tissue from any proliferation medium were placed in 5 ml of liquid Glitz0 and gently suspended using a pipette tip (as described above). The resulting suspension was poured onto a qualitative filter paper disc (Grade MS 170MM) in a Buchner funnel and a short vacuum pulse was applied to anchor the cells (Klimaszewska and Smith 1997). The filter paper, covered with a layer of cells, was then transferred to a Glitz maturation medium (Table 1) without 2,4-D and BA but containing 60 g L−1 sucrose, 60 µM Trans-ABA (abscisic acid, Duchefa, Netherlands) and 8 g L−1 gellan gum (Phytagel®, Sigma). Cultures were incubated at 24 °C in the dark. Mature cotyledonary SEs were harvested after approximately 8–12 weeks. Embryos were placed onto a Glitz-based germination medium without plant growth regulators, containing 20 g L−1 sucrose and 3.5 g L−1 gellan gum, cultured at 24/18 °C (day/night temperature, respectively) under a 16 h photoperiod at a light intensity of 90 µmol m−2 s−1 using fluorescent lights. After 4–6 weeks, plantlets were transferred directly to a nursery propagation facility for acclimatization.
Data collection and statistical analysis
Approximately 3 weeks after the transfer of responding explants to proliferation media, all potential cell lines were registered and given a cell line number. Lines were classified after 9 weeks according to their phenotype under the binocular microscope, using the following classifications; embryogenic tissue (containing multicellular embryo initials with multiple suspensor cells), putative embryogenic tissue (containing small embryo initials and some suspensor cells), a mix of embryogenic tissue and callus, and callus.
The data for cell line initiation, the influence of collection date and overall treatment effects on cell line initiation and proliferation data collected from established cell lines for relative FM gains (%) were analyzed using PROC MEANS of the SAS software package (SAS Institute Inc., 2010, SAS Users Guide, Version 9.1, Cary, NC, USA). Following ANOVA, treatment and cell line means were compared using the Tukey multiple range test option.
Results
Initiation experiments
Experiment 1(2008/2009): initiation medium effect (Glitz vs BM)
Douglas-fir ZE development was rapid during the 2008–2009 season, with only two of the five trees sampled having pro-embryos present in the 21st November collection, but 100% mature cotyledonary embryos present in all five trees by the 6th of January (Table 2). Embryo excision was difficult and the lack of full seed in some OP families made progress slow. The data collected from the embryo development scoring indicated a steep decline in full seed through the collection period going from 100 to 50% full seed over the four collections (Table 2). Some cones were infested with Megastigmus spermotrophus Wachtl grubs. Despite these factors, contamination of explants from collections 2 and 3 was low with only three embryos (0.6%) over the whole initiation Experiment 1 affected. Collection 1 was not included in the initiation experiment (Table 3) due to the low development score of ZEs.
All OP families tested gave rise to embryogenic tissue, with an overall initiation rate on Glitz of 42.9% at collection 3 (Table 3). In contrast, the BM1 medium developed by Gupta et al. (2004) failed to initiate any embryogenic cell lines.
Analysis of variance results of the overall treatment, family and collection date means showed significant collection and treatment effects (p ≤ 0.0001). Family and family by treatment interaction effects were not significant (Table 4). When collections were compared, collection 3 was significantly better (p ≤ 0.05) than collections 2 and 4 (Table 3) for the initiation of embryogenic cell lines.
On Glitz, three of the five OP seed families had an initiation rate of 58.3% at the best collection date of 18th December (Table 3). Initiation rates from the collection made on the 6th of January 2009 were reduced (5.5–22.2%), most likely a result of the advanced stage of development of the ZEs (Table 2). However, it should be noted that on the Glitz medium some embryogenic cell lines were formed, and in fact, this was the only collection date where sampled seeds resulted in embryogenic cell lines for OP family 5 (22.2%, Table 3).
Phenotypic classification of the 52 cell lines obtained after 9 weeks on initiation media (Table 5) showed that 19 (36.5%) were clearly embryogenic, 7 (13.5%) were putative embryogenic lines, 4 (7.7%) were calli and 22 (42.3%) were a mix of embryogenic tissue and calli. The earliest collection date (2–6th December) produced 30% of mixed cultures, which was the lowest number of all collections. In collection 3 (18th December), the occurrence of mixed cultures had increased slightly to 37% and by the final collection date (6th January), the percentage of initiated lines, which were mixed cultures, had reached 60% of the total lines initiated from that collection (data not presented).
Experiment 2 (2011/2012): initiation media comparison (Glitz vs MSG) and explant types (intact megagametophytes vs excised zygotic embryos)
Two collections of cones (21st December and 30th December) were made during Experiment 2 (2011–2012) for each of the five selected OP families, with the exception of family 11. Cones of this family collected on the 21st December contained megagametophytes which were withered and yellow in colour. One of the cones contained only a small number of seeds and the cones were infested with grubs of Megastigmus spermothrophus Wachtl. Due to the poor health of cones from this family, it was decided not to sample it a second time. Family 15 also contained unhealthy megagametophytes, all showing some degree of withering, probably due to tree and/or orchard water stress (regional drought conditions in December 2011). However, it was decided to sample this family on the second collection date as a significant rainfall occurred in the area between the two collection dates. One cone from family 14 contained lots of seed but megagametophytes were glassy and contained very immature embryos, with an average embryo development score of only 1.6 (Table 2). The remaining families (12 and 13) contained healthy and more developed embryos compared to the other three families, giving average embryo development scores of 3.6 and 3.0, respectively. By the second collection date, only 9 days after the first collection, embryos from all families had significantly developed, with an overall average embryo development score of 6.0 (Table 2).
Analysis of variance results of the overall family, collection, media and treatment effects showed significant family and collection effects (p ≤ 0.005) and highly significant medium and treatment effects (p ≤ 0.0001) (Table 6). Treatment by family interaction effects was not significant.
When all families and treatments were combined, collection two was significantly better than collection one for initiation (≤ 0.005) with average initiation percentages of 18.2 and 8.4%, respectively (Table 7). This is most likely to be a reflection of the higher average developmental scores on the second collection date, 6.0 and 2.4, respectively (Table 2).
For all families over both collection dates, treatment 7 (GlitzB ZE) produced significantly more cell lines than all other treatments (Table 7). Treatment 5 (Glitz ZE) was the next best, being significantly better than all the remaining treatments. Both MSG medium and the use of whole megagametophytes (irrespective of medium) were unfavourable treatments.
Phenotypic classification of the 96 cell lines initiated (Table 5) showed that 18 (18.7%) were clearly embryogenic, 45 (46.9%) were putative embryogenic lines, 5 (5.2%) were calli and 28 (29.2%) were a mix of embryogenic tissue and calli. Of these 28 mixed cultures, 26 originated from Glitz-based media, with 16 (61.5%) from Glitz (higher 2,4-D) and 10 (38.5%) from GlitzB (lower 2,4-D). Of the 28 mixed cultures, 9 (32% of the cultures) originated from collection 1 and 19 (68% of the cultures) from collection 2.
Experiment 3 (2012/2013): culture of excised ZEs on GlitzB
Cones from trees in a seed orchard in Amberley, North Canterbury, were originally sampled for this experiment 3 (2012–2013); however, when cones were opened they had very poor seed set, believed to be due to pruning of the hedges during the winter. We were able to obtain cones from another seed orchard in Otago. However, by the time this was organized, it was mid-January, later than we would normally sample this species in New Zealand. This was reflected in the high average embryo development scores of the five families (Table 2, averaging 6.6), which was, however, a development score quite similar to the optimal average score for initiation during Experiment 2 (6.0, Table 2).
The percentage of cell lines initiated and continuing to proliferate ranged from 60.6 to79.6% (overall 69.3% initiation rate, Table 8). The initiation rate on GlitzB was confirmed to be high and robust among five independent families, as previously observed during the 2011–2012 season using GlitzB.
Phenotypic classification of the 212 cell lines (Table 5) showed that 55 (25.9%) were clearly embryogenic, 80 (37.7%) were putative embryogenic lines and 77 (36.3%) were a mix of embryogenic tissue and calli. Interestingly, no initiated lines reversed towards calli after 3–4 months of proliferation.
Considering our best data from each initiation season, ZE development scores from 3.4 to 6.6 were found suitable for initiation in Douglas-fir. These scores correspond to early embryogenesis stages 3 up to precotyledonary embryos. Developmental scores less than 2.5 (proembryo stages) were too early for optimal initiation rate. Initiation rate significantly decreased at the cotyledonary stage for most families (score of 8.0, see Tables 2, 3).
A synthesis of phenotype characterization of each of the initiated lines obtained after the three initiation seasons is presented in Table 5. Across experiment 1 (mainly lines from Glitz) and experiments 2 and 3 (mainly lines from GlitzB), the initiation rate of embryogenic and putative embryogenic lines increased (50.0 vs. 63.6–65.6%, respectively), whereas mixed cultures (embryogenic tissue and calli) decreased (42.3 vs. 29.2–36.3%) as well as the percentage of lines which apparently reversed towards calli following multiplication (7.7 vs. 0–5.2%).
Proliferation experiment: effect of carbohydrate source, tissue preparation method and cell density for proliferation of Douglas-fir embryogenic lines on Glitz basal media
Analysis of variance results of the overall cell line and treatment means showed significant cell line effects (p ≤ 0.05) and treatment effects (p ≤ 0.0005) and significant cell line by treatment interaction effects (p ≤ 0.0001, Table 9) .
Across all cell lines, there were few significant differences between treatments (as shown by Tukey’s range test, Table 10); the general trend however shows treatment 5 (Malt-1-100) to be a useful treatment overall, giving the highest mean relative FM gains for three out of the four cell lines, and explains the significant treatment effects in the ANOVA results, as the only real significant differences were between treatment 5 and treatments 3 and 4. Plating 100 mg of tissue instead of 200 mg yielded similar results. Although also not significant when cell lines were combined, tissue preparation method 1 (vigorous treatment of tissue) tended to yield higher FM gains compared with method 2 (gentle treatment of tissue) when comparing otherwise identical treatments.
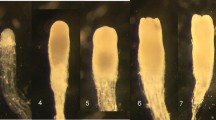
Acetocarmine staining showed that proliferation on medium containing maltose (GlitzM) instead of sucrose (Glitz) consistently increased SE differentiation of all cell lines tested (Fig. 1). On Glitz, suspensors were poorly organized and/or SE were quite small in size. On GlitzM, early embryos were comparatively better organized and well-developed, with large embryo heads and suspensors, especially in the case of lines 29-2 and 110-3. For these two lines, increased embryo differentiation was concomitant with increased FM gains on GlitzM (Malt-1-100) compared to Glitz (Suc-1-100).
Somatic plant recovery from cryopreserved lines
Samples of 14 initiated cell lines on Glitz were recovered after cryopreservation using Pinus radiata protocols (see Materials and Methods). Of these 14 cell lines, 8 continued to proliferate well enough to test the standard maturation protocols used for Pinus radiata (see Materials and Methods). All of these eight cell lines produced mature SEs within 10–12 weeks with varying success of quality and quantity (data not shown). These cell lines had not been tested for maturation potential prior to being cryopreserved. Most cotyledonary SEs were able to germinate within 2 weeks using Glitz germination medium (Fig. 2).
Discussion
We have successfully adapted recently developed protocols for the initiation of embryogenic tissue of P.radiata (Hargreaves et al. 2009, 2011) to Douglas-fir immature zygotic embryos, using simple techniques and media available in the public domain.
Immature seed sampling from tested families in Douglas-fir
The destructive examination of seeds of each family at each sampling year supported the anecdotal information that the seed production of Douglas-fir can be problematic. Douglas-fir forms cones but these may not always receive sufficient pollen to produce filled seed in all circumstances, resulting in abnormally developed seed, or no seed. In a study carried out on five Douglas-fir trees from one 15-year-old seed orchard located in British Columbia, an average seed production was 39%, this major loss of seed was attributed to insufficient pollen in the ovules (Owens et al. 1991). Seed predation by Megastigmus spermotrophus infestation can also affect the number of seeds at maturity, although this does vary among sites and seasons (Bain 1977). In the work presented here, M. spermotrophus grubs were observed during the 2008–2009 and 2011–2012 seasons. In the 2008–2009 collection period, there was a serious decline from an assumed 100% full seed cone at the first collection, to approximately half that amount when the cones were dissected at the time of fully developed ZE (cotyledonary stage). It was difficult to get sufficient numbers of cones from the trees available for sampling in Rotorua and the numbers of full seed per cone varied, possibly due to the position of the cones on the trees and sub-optimal weather conditions at time of pollination. Apart from lack of filled seed, environmental conditions can also have a huge effect on the quality of seed within the cones, which can subsequently affect the ability of the explant to produce embryogenic tissue. This was illustrated during the 2011–2012 collection, where two of the OP families had a large number of withered and yellow megagametophytes, most likely due to water stress caused by regional drought conditions during this season (data not shown). These families, as well as family 14, had slower ZE development compared to the other sampled families. It may be anticipated that different ZE development scores could result in the need for different initiation conditions.
Precotyledonary embryo as optimal developmental stage for initiation
Three years of initiation experiments have culminated in a treatment giving initiation rates ranging between 60.6 and 79.6% in five OP families (2012–2013 season). The average initiation rate (69.3%) during this season indicated high and sufficient genotype capture at this step, similar to what is obtained in P. radiata (70%, Hargreaves et al. 2009) or P. pinaster (77%, Trontin et al. 2011). Surprisingly, these results were from OP trees/cones containing well-developed ZEs, with an average embryo development score of 6.6. This corresponds to ZEs at the precotyledonary to early cotyledonary stages. Traditionally, the optimal time reported for embryo selection in Douglas-fir is 4–6 weeks after fertilization, when the apical dome of a ZE begins to form, but before the first appearance of cotyledonary primordia (precotyledonary stage; Durzan and Gupta 1987; Gupta et al. 1994; Pullman et al. 2005, 2009a). Of course, it is possible that using ZEs that were less developed may have resulted in even higher initiation rates, but in 2012–2013 only one collection was made. The best time for an optimal initiation experiment seems, however, very short and corresponds to less than 9 days as illustrated in 2011–2012. During the 2011–2012 season, collection 2, two families (12 and 13) had similar or slightly more advanced development scores (7.6 and 7.2, respectively), as was observed in 2013 (6.0–7.6). On GlitzB, these families produced good initiation rates (50–58%), these were however, lower than during 2012–2013 (61–79%), supporting the conclusion that the precotyledonary stage (development score around 6) is more optimal compared to the early cotyledonary stage (development score around 7). Other factors besides development scores of the ZEs, may be involved in this improved initiation result, such as family effect (different families were used in 2011–2012 from those used in 2012–2013). It is noteworthy that all five independent families tested in 2012–2013 produced similar high initiation rates. The proliferation methods used with the lines initiated in 2013 (repeated suspension in Glitz 0 of the embryogenic portion of the tissue and replating on GlitzB and/or GlitzM, see Materials and Methods) are highly likely to have contributed to the increase in numbers of mixed culture lines remaining embryogenic.
The superiority of Glitz medium formulation for initiation of embryogenic cell lines
The most successful medium formulation over 3 years of initiation experiments carried out during this work was, by a significant margin, Glitz. This is a modified Litvay medium (Litvay et al. 1985) recently found to be very successful for the initiation of embryogenic tissue in Pinus radiata (Hargreaves et al. 2009, 2011). Modified Litvay medium has been used successfully for the initiation of embryogenic cell lines in a number of other pines, especially in combination with a reduction in plant growth regulators (Percy et al. 2000; Klimaszewska et al. 2001). A modified Litvay medium was also recently used to initiate embryogenic lines in Douglas-fir (Kong and von Aderkas 2011), although this paper focussed on cryopreservation of immature SEs and did not report on the success of this medium for embryogenic tissue initiation. BM medium formulation has been successfully used by other researchers for Douglas-fir initiation in North America, initially named WTC (Pullman and Gupta 1994; Gupta 1996) and later BM1 (Gupta et al. 2004), and with slight alterations to the formulation, 121 (Pullman et al. 2005, 2009a). Somewhat surprisingly, BM1 was unsuccessful with New Zealand plant material in our laboratory conditions, irrespective of ZE developmental stage. We cannot exclude any unidentified difference in the medium composition that is classically seen when methods are transferred among different laboratories (Park et al. 2006). It should, however, be noted that the ZE dissection method employed in the present work was different than that of these authors, who used BM1 medium with ZE suspensors still attached to megagametophytes. Interestingly, recent work by Pullman et al. (2009a) indicated improved results on BM1 medium if a range of supplements were added. These include abscisic acid, pyruvic acid and brassinolide. Even with these supplements and rigorous embryo screening for health, initiation rates averaged 40–57%. The preliminary SE initiation results obtained on a Glitz medium, in the first season of sampling in 2008–2009 of 25–58% (42.9% overall) at the optimal collection date, which suggested the superiority of Glitz and hence the BM1 medium was not included in the next experiments. The Glitz medium was tested again on a large scale in the 2011–2012 season (with five new families) and similar SE initiation success was obtained, ranging from 17 to 67% (43.8% overall).
Results from the 2011–2012 season confirmed the superiority of the Glitz and GlitzB formulations for the initiation of Douglas-fir embryogenic tissue, with overall SE initiation rates of 31.1 and 41.3%, respectively. When ZEs were excised from the megagametophytes, 84% of the cell lines initiated on these medium formulations. The second medium tested was MSG (Becwar et al. 1987), a modified Murashige and Skoog (MS) medium (Murashige and Skoog 1962), the same medium as used by Nagmani et al. (1992) in their study of Douglas-fir initiation. These authors carried out a comprehensive study using 7055 intact pre- and post-fertilization ovules and 5693 isolated precotyledonary and cotyledonary ZEs on a variety of media, including MS medium. None of the intact ovules and only 4 of the 2813 isolated precotyledonary ZEs produced embryogenic tissue, and 3 of these 4 were from the same mother tree. This study, combined with our results with MSG that were in the range 0–33% initiation for different families at the best collection date, suggests that MS is not a good medium option for Douglas-fir somatic embryogenesis initiation. The first published paper on somatic embryogenesis in Douglas-fir similarly reported a quite low initiation rate of 20–25% at the best developmental stages (Durzan and Gupta 1987), using a MS-based medium, BM-1, not to be confused with BM1 used by Gupta and Pullman mentioned above (Gupta et al. 2004).
Excised zygotic embryo as superior explant for initiation
The 2011–2012 sampling season was the first time we tested intact megagametophytes as an explant for initiating embryogenic tissue in Douglas-fir. Results clearly confirmed our expectations that excised ZEs would be a superior explant, especially in combination with Glitz medium. The average initiation rates for different Glitz formulations (Glitz, GlitzB) in combination with dissected ZE were significantly higher than when intact megagametophytes were used. This explant (ZE) and medium (Glitz) combination was found to be far superior for initiation of P. radiata embryogenic tissue, with an overall average initiation rate of 55% compared to the previous standard initiation protocol of 16% (Hargreaves et al. 2009). The majority of published papers and patents reported the use of excised ZEs still attached to the megagametophytes as the best explant for initiation of embryogenic tissue of Douglas-fir (Durzan and Gupta 1987; Gupta et al. 1994, 2004; Pullman et al. 1994, 2005, 2009a). Although none of these authors provided an explanation for why this is done, as opposed to removing the ZE completely, we assume that the belief is that the ZEs would receive growth regulators and/or nutrition from fluids accumulating in the corrosion cavity (Carman et al. 2005) that will assist in the initiation of embryogenic tissue. Fully excised ZEs gave good results with New Zealand plant material in our experimental conditions, suggesting that the megagametophyte effect on initiation rate from the ZEs would be quite low or even negative, as observed in our experiments when intact megagametophytes were used.
Removing the immature ZE from the megagametophyte entirely has allowed us to avoid a harsh disinfection protocol. Typically, disinfection protocols for Douglas-fir immature seed include commercial bleach or detergent, followed by a treatment with hydrogen peroxide, and sometimes mercuric chloride (Durzan and Gupta 1987; Nagmani et al. 1992; Gupta et al. 1994, 2004; Pullman et al. 2005, 2009a). For the majority of work presented here, the cones were simply submerged in 70% ethanol for approximately 30 min, and then seeds were removed and briefly dipped in 70% ethanol prior to embryo extraction. Contamination rates were less than 1% in all experiments. This method has also been proven to be very successful for maritime pine (Lelu-Walter et al. 2006) and hybrid larch (Lelu-Walter and Pâques 2009).
The high incidence of initiated cell lines containing a mixture of embryogenic tissue and callus
An interesting aspect of our first initiation test with New Zealand Douglas-fir was the high incidence of cell lines containing embryogenic tissue mixed with callus; i.e. 42.3% during the 2008–2009 season. This has not been observed with P. radiata using the same media and techniques (Hargreaves et al. 2009). There were also indications that some of these mixed cultures, after 9 weeks of subculture, could lose all embryogenic tissue, thus becoming callus alone. These cell lines were very difficult to maintain and proliferate and they required frequent transfers (every 7 days). It is highly likely that some initiated lines may have been lost due to the fact that they were not all transferred in a timely manner. This may explain why a significant percentage of initiated lines in 2008–2009 (7.7%) were later phenotyped as calli. Durzan and Gupta (1987) found that Douglas-fir embryonal-suspensor masses (ESMs) turned into calli when subculture was delayed to more than 15 days. In maritime pine, it was also reported that weekly subcultures rather than bi-weekly were required to delay the “ageing” of embryogenic cultures, which became unproductive, non-embryogenic versions of original lines (Breton et al. 2005). It is possible that callus is derived from differentiating embryo initials that reach a point of development where they cease cleavage polyembryony but have insufficient environmental stimuli (for example, abscisic acid) to continue differentiating. Instead, the plant growth regulators in the proliferation medium (BA and 2,4-D) encourage callogenesis. The production of mixed cultures continued through all years of initiation, ranging from 29.2 to 42.3% of all cell lines initiated.
Durzan and Gupta (1987) mentioned in their paper that callus was obtained at the same time as the SE tissue on their MS-modified medium. However, from the figure presented by the authors, it appeared to be contained within an area of the embryogenic tissue, rather than being mixed within the embryogenic cells, as we have seen in our work (Fig. 3), potentially making it easier to separate. These authors mentioned the separation of the embryogenic tissue from the callus on the medium, but there was no discussion of the continued maintenance of these lines and whether the callus was a continuing issue. Jiang (1991) found that embryogenic tissue initiated from immature Douglas-fir ZEs turned brown by the third and fourth transfers. After 1–3 weeks, a piece of white callus often formed on the brown callus and the production of stable embryogenic cell lines was obtained by separating small sections of this embryogenic tissue from the mass of brown calli. Other authors make no mention of the occurrence of these mixed cultures on BM-based media, such as BM1 tested in our study. These media contain very high concentrations of auxins and cytokinins due to the presence of activated charcoal in the medium which suppresses plant growth regulator (PGR) activity (Gupta et al. 1994; Pullman et al. 2005, 2009a). It is therefore very difficult to compare the concentrations of PGR with those contained in Glitz media, which contain no activated charcoal.
The occurrence of mixed cultures was lower in experiments 2 and 3 (29.2–36.3%) compared to experiment 1 (42.3%). There was concomitantly a lower occurrence of callus lines (0–5.2 vs. 7.7%) and also an increase in the average initiation rates of embryogenic lines under the best conditions (50.9–69.3 vs. 42.9%). This suggests that callus occurrence decreased as the initiation conditions gradually improved from the Glitz (Experiments 1 and 2, 2008–2012) to GlitzB protocol (Experiments 2 and 3, 2011–2013). Klimaszewska et al. 2001 showed that when PGRs were decreased in Litvay medium, initiation of embryogenic tissue in Pinus strobus was significantly increased. Another possible explanation is that callus occurrence is related to development score of the initial ZE explant, as a global increase across experiments (2008–2013) from 3.4 to 6.6 was observed. Further experiments with different families sampled at different development stages of ZEs are needed to support such a hypothesis.
In the 2011–2012 collection season, explants were placed on Glitz media containing varying 2,4-D concentrations. Of the cell lines which were initiated, only 28 (29.2%) were mixed cultures, considerably less than the previous initiation experiment. This reduction appears to be mainly due to the lowering of 2,4-D in the medium, with 61.5% of these mixed cultures originating from Glitz containing the higher level of auxin (1 mg L−1 2,4-D) and only 38.5% originating from GlitzB (0.5 mg L−1 2,4-D). Hong et al. (1992) found that higher levels of 2,4-D in initiation media led to the formation of calli, especially from more mature ZEs. Further modifications of Glitz medium at the initiation and proliferation stages may be required to maintain tissue in a state of continuous embryogenic tissue production.
The beneficial effect of maltose on embryogenic cell line proliferation and development of immature SEs
During proliferation of Douglas-fir embryogenic tissue, the use of maltose in combination with Glitz, although not significant, generally resulted in better growth compared to sucrose as a carbohydrate source. Where vials containing cells in liquid media were shaken to disperse cells, followed by vigorous and repeated drawing in and out of the pipette tip, before being dispensed onto the surface of the semisolid medium, higher FM gains were generally achieved than with the more gentle treatment, although this was not statistically significant. This difference was especially conspicuous in combination with maltose for three out of the five lines. This could be due to the fact that maltose promoted growth and organization of early SEs compared to sucrose in all of the lines proliferated on Glitz medium (Fig. 1). Particularly well-developed, organized immature SEs on proliferation medium containing maltose were observed for some lines. A similar effect has been reported in a number of other conifer species. Gupta (1996) recommended the use of maltose for a wide range of conifers including Douglas-fir, reporting that it resulted in the early stage SEs growing in size and vigour to advanced early staged SEs on a variety of media. In Scots pine SEs, the size of the embryo increased when maltose was used instead of sucrose in MSG and DCR media (Keinonen-Mettala et al. 1996). In maritime pine, maltose was found to slightly improve early SE development when used instead of sucrose in DCR medium (Breton et al. 2005) (Fig. 2).
In conclusion, medium and protocols already used for P. radiata have been shown to be successful for the induction and proliferation of Douglas-fir embryogenic cell lines. These include (1) simplified disinfection of explants, avoiding the use of prolonged exposure to harsh chemicals; (2) the use of a modified Litvay medium (Glitz) with a lower concentration of 2,4-D (GlitzB); (3) the culturing of ZEs, which was shown to be far superior explants compared to whole megagametophytes; (4) and the use of maltose rather than sucrose in the medium during the proliferation stage, generally resulting in greater FM gains in all cell lines and improved the development of immature SEs in some lines. These modifications greatly reduced the number of cell lines containing a mix of embryogenic tissue and calli.
We have also shown that it is possible to cryopreserve and then produce mature SEs from re-grown lines. SEs were successfully germinated on Glitz medium, however, further work is required to improve the SE yield and quality. Future studies will include optimisation of the maturation medium and protocols and the conversion of mature SEs to axillary shoot cultures to take full advantage of the best of both of these propagation systems, allowing cryopreservation of valuable cell lines, high multiplication of good quality SEs and synchronised development of shoot cultures prior to adventitious root induction and transfer to the nursery.
Author contribution statement
CR contributed to experimental design, conducted initiation and proliferation experiments, carried out cryopreservation and maturation testing and wrote the draft of the manuscript. CH contributed to experimental design, conducted initiation experiments and reviewed the manuscript. JFT contributed to experimental design, conducted some proliferation experiments and contributed to the writing of the manuscript. MALW contributed to experimental design and reviewed the manuscript.
Abbreviations
- ABA:
-
Abscisic acid
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- BA:
-
6-Benzylaminopurine
- BM1 :
-
Gupta basal medium (Gupta et al. 2004)
- DMSO:
-
Dimethylsulfoxide
- FM:
-
Fresh mass
- Glitz:
-
Litvay medium (Litvay et al. 1985, modified by; Hargreaves et al. 2009)
- MSG:
-
Becwar medium (Becwar et al. 1987)
- OP:
-
Open pollinated
- PGR:
-
Plant growth regulator
- SE:
-
Somatic embryo
- ZE:
-
Zygotic embryo
References
Bain J (1977) Megastigmus spermotrophus Wachtl (Hymenoptera: Chalcidoidea: Torymidae). New Zealand Forest Service, Forest Research Institute, Forest and Timber Insects in New Zealand No. 14
Bastien J-C, Sanchez L, Michaud D (2013) Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco). Chapter 7. In: Pâques Luc E., 10427J, dir., Forest tree breeding in Europe. Current state-of-the-art and perspectives. Managing forest ecosystems, vol 25. Springer, Dordrecht, pp 325–369. doi:10.1007/978-94-007-6146-9_7
Becwar MR, Wann SR, Johnson MA, Verhagen R, Feirer, Nagmani R(1987) Development and characterization of in vitro embryogenic systems in conifers.Tech Pap Ser Inst Paper Chem, Appleton, WE, USA, No. 258, pp 1–17
Breton D, Harvengt L, Trontin J-F, Bouvet, Favre JM (2005) High subculture frequency, maltose-based and hormone-free medium sustained early development of somatic embryos in maritime pine. Vitro Cell Dev Biol Plant 41(4):494–504
Carman JG, Reese G, Fuller RJ, Ghermay T, Timmis R (2005) Nutrient and hormone levels in Douglas-fir corrosion cavities, megagametophytes, and embryos during embryony.Can J For Res 35:2447–2456
Dungey HS, Low CB, Lee J, Miller MA, Fleet K, Yanchuk AD (2012) Developing breeding and deployment options for Douglas-fir in New Zealand: breeding for future forest conditions. Silvae Genet 61(3):104–115
Durzan DJ, Gupta PK (1987) Somatic embryogenesis and polyembryogenesis in Douglas-fir cell suspension cultures. Plant Sci 52(3):229–235
Find JI, Floto F, Krogstrup P, Moller JD, Norgaard JV, Kristensen MMH (1993) Cryopreservation of an embryogenic suspension culture of Picea sitchensis and subsequent plant regeneration. Scand J For Res 8(1–4):156–162
Gupta PK (1996) Method for reproducing conifers by somatic embryogenesis using a maltose enriched maintenance medium: US Patent No. 5,563,061
Gupta PK, Durzan DJ (1987) Biotechnology of somatic polyembryogenesis and plantlet regeneration in loblolly pine. Bio Technol 5:147–151
Gupta PK, Pullman GS (1996) Method for reproducing douglas-fir by somatic embryogenesis. US Patent No 5,482,857. US Patent and Trademark Office, Washington, DC
Gupta PK, Dandekar AM, Durzan DJ (1988) Somatic proembryo formation and transient expression of a luciferase gene in Douglas fir and loblolly pine protoplasts. Plant Sci 58(1):85–92
Gupta P, Dandekar AM, Durzan DJ (1989) Genetic transformation system in Douglas-fir Pseudotsuga menziesii. Applications of biotechnology in forestry and horticulture. Springer, US, pp 339–347
Gupta PK, Timmis R, Timmis, Carlson, Grob J, Welty E (1994) Plantlet regeneration via somatic embryogenesis in Douglas-fir (Pseudotsuga menziesii). TAPPI biological sciences symposium proceedings, pp 35–39
Gupta P, Holmstrom D, Budworth D (2004) Embryogenic culture initiation of Douglas-fir by maltose.US Patent Application No. 20040237130
Hargreaves CL, Grace LJ, Holden DG (2002) Nurse culture for efficient recovery of cryopreserved Pinus radiata D. Don embryogenic cell lines. Plant Cell Rep 21(1):40–45
Hargreaves CL, Reeves CB, Find JI, Gough K, Josekutty P, Skudder DB, Van der Maas SA, Sigley MR, Menzies MI, Low CB, Mullin TJ (2009) Improving initiation, genotype capture, and family representation in somatic embryogenesis of Pinus radiata by a combination of zygotic embryo maturity, media, and explant preparation. Can J For Res 39(8):1566–1574
Hargreaves CL, Reeves CB, Find JI, Gough K, Menzies MI, Low CB, Mullin TJ (2011) Overcoming the challenges of family and genotype representation and early cell line proliferation in somatic embryogenesis from control-pollinated seeds of Pinus radiata. N Z J For Sci 41:97–114
Hong L, Boulay M, Gupta PK, Durzan DJ (1992) Variations in somatic polyembryogenesis: Induction of adventitious embryonal-suspensor masses on developing Douglas-fir embryos. In Ahuja MR (ed) Woody plant biotechnology, vol 210. Plenum Press, New York, pp 105–121
Jiang L (1991) Somatic embryogenesis and genetic transformation in Douglas-fir. PhD Thesis, The University of British Columbia, p 133
Keinonen-Mettala K, Jalonen P, Eurola P, Von Arnold S, Von Weissenberg K (1996) Somatic embryogenesis of Pinus sylvestris. Scand J For Res.11:242–250
Klimaszewska K, Smith DR (1997) Maturation of somatic embryos of Pinus strobus is promoted by a high concentration of gellan gum. Physiol Plant 100:949–957
Klimaszewska K, Park Y-S, Overton C, Maceacheron I, Bonga JM (2001) Optimized somatic embryogenesis in Pinus Strobus L.. Vitro Cell Dev Biol Plant 37:392–399
Klimaszewska K, Hargreaves CL, Lelu-Walter M-A, Trontin J-F (2015) Advances in conifer somatic embryogenesis since year 2000. In: Germanà MA, Lambardi M (eds) In vitro plant embryogenesis in higher plants. Methods in molecular biology. Springer, Berlin, pp 131–166
Kong L, von Aderkas P (2011) A novel method of cryopreservation without a cryoprotectant for immature somatic embryos of conifer. Plant Cell Tiss Organ Cult 106:1 pp115
Kong L, Denchev P, Radley R, Lobatcheva II, Attree SM (2011) Method of culturing conifer somatic embryos using S (+)-abscisic acid. US Patent No. 7,951,594. US Patent and Trademark Office, Washington, DC
Ledgard N, Knowles K, De Lar Mare P (2005) Douglas-fir—the current New Zealand scene. NZ J For 50:13–16
Lelu-Walter M-A, Pâques LE (2009) Simplified and improved somatic embryogenesis of hybrid larches (Larix × eurolepis and Larix × marschlinsii). Perspectives for breeding. Ann For Sci 66:104
Lelu-Walter MA, Bernier-Cardou M, Klimaszewska K (2006) Simplified and improved somatic embryogenesis for clonal propagation of Pinus pinaster (Ait.). Plant Cell Rep 25:767–776
Lelu-Walter MA, Thompson D, Harvengt L, Sanchez L, Toribio M, Pâques LE (2013) Somatic embryogenesis in forestry with a focus on Europe: state-of-the-art, benefits, challenges and future direction. Tree Genet Genomes 9:883–899
Litvay JD, Verma DC, Johnson MA (1985) Influence of a loblolly pine (Pinus taeda L.). Culture medium and its components on growth and somatic embryogenesis of the wild carrot (Daucus carota L.). Plant Cell Rep 4(6):325–328
Low C, Ledgard N, Shelbourne T (2012) Early growth and form of coastal provenances and progenies of Douglas-fir at three sites in New Zealand. N Z J For Sci 42:143–160
Michaud D, Bastien JC, Sanchez L, Bouvet A, Eisner N (2014) Towards a Douglas fir breeding population for long term. IUFRO Forest Tree Breeding Conference August 25–29, 2014, Prague, Czech Republic, p 34
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nagmani R, Johnson MA, Dinus RJ (1992) Effect of explant and media on initiantion, maintenance, and maturation of somatic embryos in Pseudotsuga menziesii (Mirb.) Franco (Douglas-fir). In: Ahuja MR (ed) Woody plant biotechnology, vol 210. Plenum Press, New York, pp 171–178
Owens JN, Blake MD (1985) Forest tree seed production: a review of the literature and recommendations for future research. Information report PI-X-53. Petawawa National Forestry Institute
Owens JN, Colangeli AM, Morris SJ (1991) Factors affecting seed set in Douglas-fir (Pseudotsuga menziesii). Can J Bot 69:229–238
Park YS, Lelu-Walter MA, Harvengt L, Trontin JF, MacEacheron I, Klimaszewska K, Bonga JM (2006) Initiation of somatic embryogenesis in Pinus banksiana, P. strobus, P. pinaster, and P. sylvestris at three laboratories in Canada and France. Plant Cell Tiss Organ Cult 86(1):87–101
Percy RE, Klimaszewska K, Cyr DR (2000) Evaluation of somatic embryogenesis for clonal propagation of western white pine. Can J For Res 30:1867–1876
Porth I, El-Kassaby YA (2014) Current status of the development of genetically modified (GM) forest trees world-wide: a comparison with the development of other GM plants in agriculture. CAB Rev 9:No. 008
Pullman GS, Gupta PK (1994) Method for reproducing conifers by somatic embryogenesis using mixed growth hormones for embryo culture. US Patent number 5,294,549
Pullman GS, Johnson S, Van Tassel S, Zhang Y (2005) Somatic embryogenesis in loblolly pine (Pinus taeda) and Douglas fir (Pseudotsuga menziesii): Improving culture initiation and growth with MES pH buffer, biotin, and folic acid. Plant Cell Tiss Organ Cult 80(1):91–103
Pullman G, Johnson S, Bucalo K (2009a) Douglas fir embryogenic tissue initiation. Plant Cell Tiss Organ Cult 96(1):75–84
Pullman GS, Chase KM, Skryabina A, Bucalo K (2009b) Conifer embryogenic tissue initiation: improvements by supplementation of medium with d-xylose and d-chiro-inositol. Tree Physiol 29(1):147–156
Shelbourne CJ A, Low CB, Gea LD, Knowles RL (2007) Achievements in forest tree genetic improvement in Australia and New Zealand 5: genetic improvement of Douglas-fir in New Zealand. Aust For 70(1):28–32
Timmis R, Grob JA, Gupta PK, Rayfield SD (2011) Methods for increasing germination vigor by early singulation of conifer somatic embryos. US Patent No. 7,964,404. US Patent and Trademark Office, Washington, DC
Trontin J-F, Reymond I, Quoniou S, Canlet F, Debille S, Bruneau G, Harvengt L, Lelu-Walter M-A, Teyssier C, Le Metté C, Vallance M, Label P (2011) An overview of current achievements and shortcomings in developing Maritime pine somatic embryogenesis and enabling technologies in France. In: Park YS, Bonga JM, Park SY, Moon HK (eds) Proceedings of the IUFRO Working Party 2.09.02 conference, August 18–21, 2010 (Suwon, South Korea), pp 100–102
Acknowlegements
We thank Christina Maher for media preparation, Keiko Gough and Barbara Geddes for technical assistance, Charlie Low and Jaroslav Klapste for their statistical advice and Proseed and Ernslaw one’s ‘Dusky’ forest seed for supply of immature cones. This research was partially funded by Future Forests Research Limited and the Dumont d’Urville NZ-France Science and Technology Support Programme and Core funding provided by The Ministry of Business, Innovation and Employment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist
Additional information
Communicated by K. Klimaszewska.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Reeves, C., Hargreaves, C., Trontin, JF. et al. Simple and efficient protocols for the initiation and proliferation of embryogenic tissue of Douglas-fir. Trees 32, 175–190 (2018). https://doi.org/10.1007/s00468-017-1622-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-017-1622-7