Abstract
Key message
The more stomata affect photosynthetic induction courses, the stronger the influence of leaf temperature on the induction parameters becomes. Initial stomatal conductances relative to species-specific stomatal thresholds explain seemingly contradictory response patterns.
Abstract
We analyzed the effects of leaf temperature, T leaf (at simultaneously altered leaf-to-air vapor concentration difference, Δw) on “overall” and “biochemical” photosynthetic induction as modified by stomata and in response to single, rectangular steps from darkness to saturating light. Studies were performed in sun leaves of shade-intolerant Betula pubescens Ehrh. and shade-tolerant Fagus sylvatica L. In general, induction proceeded faster with higher T leaf. This pattern was clearly visible when limitations to induction were dominated by stomata, which was the case at low initial stomatal conductance (g ini), as well as when there was a long stomatal lag time in response to the light-step (t lag) and slow stomatal opening (long time to reach 90% of full stomatal conductance, t 90%g). t lag and t 90%g became shorter with rising T leaf despite strongly increasing Δw (temperature-driven), while g ini was not affected. Species-specific thresholds, namely g ini(crit), above which halftimes of induction are no longer related to g ini, were higher in Betula (≈35 mmol m−2 s−1) and most likely not temperature related, while they decreased with T leaf (from 15 to 35 °C) in Fagus from about 30 to 10 mmol m−2 s−1. In Betula, g ini values were typically above and in Fagus typically below g ini(crit). Induction states 60 s after the light-step (IS60) rose with temperature while becoming more sensitive to g ini with higher T leaf. Induction courses which began either above or below g ini(crit) resulted in entirely different dependencies of induction halftimes on T leaf, confirming the importance of g ini(crit). This must be kept in mind when comparing different species. Implications for modeling are discussed.









Similar content being viewed by others
Abbreviations
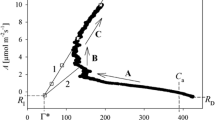
- Γ*:
-
Light-independent CO2 compensation point
- Δw :
-
Leaf/air vapor concentration difference
- A :
-
Net photosynthesis
- A ind :
-
Overall photosynthetic induction
- A max :
-
Maximum A at full photosynthetic induction
- B ind :
-
Biochemical photosynthetic induction
- C a :
-
Ambient CO2 concentration
- C i :
-
Intercellular CO2 concentration
- g :
-
Stomatal conductance to H2O
- g ini :
-
g before illumination
- g ini(crit) :
-
Stomatal threshold value in the curved region of the function t 50%A = f (gini)
- g m :
-
Mesophyll conductance to CO2
- g max :
-
g at full induction
- IS60 :
-
Induction state after 60 s of illumination
- m 100 :
-
Initial linear slope of the A/C i relationship at full induction
- PPFD:
-
Photosynthetic photon flux density
- R D :
-
Leaf respiration in darkness
- R I :
-
Leaf respiration in light
- RuBisCO:
-
Ribulose-1,5-bisphosphate carboxylase/oxygenase
- RuBP:
-
Ribulose-1,5-bisphosphate
- t :
-
Time
- t 50%A and t 90%A :
-
Time to reach 50 and 90% of A ind
- t 50%B and t 90%B :
-
Time to reach 50 and 90% of B ind
- t 90%g :
-
Time to reach 90% of g max
- t lag :
-
Lag time in stomatal response after beginning of illumination
References
Allen MT, Pearcy RW (2000) Stomatal behavior and photosynthetic performance under dynamic light regimes in a seasonally dry tropical rain forest. Oecologia 122:470–478. doi:10.1007/s004420050968
Bernacchi CJ, Singsaas EL, Pimentel C et al (2001) Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ 24:253–259. doi:10.1046/j.1365-3040.2001.00668.x
Beyschlag W, Lange OL, Tenhunen JD (1986) Photosynthese und Wasserhaushalt der immergrünen mediterranen Hartlaubpflanze Arbutus unedo L. im Jahresverlauf am Freilandstandort in Portugal. I. Tagesläufe von CO2-Gaswechsel und Transpiration unter natürlichen Bedingungen. Flora 178:409–444
Brooks A, Farquhar GD (1985) Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Estimates from gas-exchange measurements on spinach. Planta 165:397–406
Bunce JA (1996) Does transpiration control stomatal responses to water vapour pressure deficit? Plant Cell Environ 19:131–135. doi:10.1046/j.1365-3040.1997.d01-3.x
Bunce JA (2000) Acclimation of photosynthesis to temperature in eight cool and warm climate herbaceous C3 species: temperature dependence of parameters of a biochemical photosynthesis model. Photosynth Res 63:59–67. doi:10.1023/A:1006325724086
Campany CE, Tjoelker MG, von Caemmerer S, Duursma RA (2016) Coupled response of stomatal and mesophyll conductance to light enhances photosynthesis of shade leaves under sunflecks. Plant Cell Environ 39:2762–2773. doi:10.1111/pce.12841
Carmo-Silva AE, Salvucci ME (2011) The activity of Rubisco’s molecular chaperone, Rubisco activase, in leaf extracts. Photosynth Res 108:143–155. doi:10.1007/s11120-011-9667-8
Chazdon RL (1986) Light variation and carbon gain in rain forest understorey palms. J Ecol 74:995–1012
Chazdon RL, Pearcy RW (1986) Photosynthetic responses to light variation in rainforest species. I. Induction under constant and fluctuating light conditions. Oecologia 69:517–523
Douthe C, Dreyer E, Brendel O, Warren CR (2012) Is mesophyll conductance to CO2 in leaves of three Eucalyptus species sensitive to short-term changes of irradiance under ambient as well as low O2? Funct Plant Biol 39:435–448. doi:10.1071/FP11190
Ehleringer JR, Sage RF, Flanagan LB, Pearcy RW (1991) Climate change and the evolution of C(4) photosynthesis. Trends Ecol Evol 6:95–99. doi:10.1016/0169-5347(91)90183-X
Engineer CB, Hashimoto-Sugimoto M, Negi J et al (2016) CO2 sensing and CO2 regulation of stomatal conductance: advances and open questions. Trends Plant Sci 21:16–30. doi:10.1016/j.tplants.2015.08.014
Ethier GJ, Livingston NJ (2004) On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar-von Caemmerer-Berry leaf photosynthesis model. Plant Cell Environ 27:137–153. doi:10.1111/j.1365-3040.2004.01140.x
Flexas J, Diaz-Espejo A (2015) Interspecific differences in temperature response of mesophyll conductance: food for thought on its origin and regulation. Plant Cell Environ 38:625–628. doi:10.1111/pce.12476
Flexas J, Diaz-Espejo A, Galmés J et al (2007) Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ 30:1284–1298. doi:10.1111/j.1365-3040.2007.01700.x
Flexas J, Barbour MM, Brendel O et al (2012) Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Sci 193:70–84. doi:10.1016/j.plantsci.2012.05.009
Gross LJ (1982) Photosynthetic dynamics in varying light environments: a model and its application to whole leaf carbon gain. Ecology 63:84–93. doi:10.2307/1937034
Kaiser E, Morales A, Harbinson J et al (2015) Dynamic photosynthesis in different environmental conditions. J Exp Bot 66:2415–2426. doi:10.1093/jxb/eru406
Kaiser E, Morales A, Harbinson J et al (2016) Metabolic and diffusional limitations of photosynthesis in fluctuating irradiance in Arabidopsis thaliana. Sci Rep 6:31252. doi:10.1038/srep31252
Kaiser E, Kromdijk J, Harbinson J et al (2017) Photosynthetic induction and its diffusional, carboxylation and electron transport processes as affected by CO2 partial pressure, temperature, air humidity and blue irradiance. Ann Bot 119:191–205. doi:10.1093/aob/mcw226
Kirschbaum MUF, Farquhar GD (1984) Temperature dependence of whole-leaf photosynthesis in Eucalyptus pauciflora Sieb. ex Spreng. Aust J Plant Physiol 11:519–538
Kirschbaum MUF, Pearcy RW (1988) Gas exchange analysis of the fast phase of photosynthetic induction in Alocasia macrorrhiza. Plant Physiol 87:818–821. doi:10.1104/pp.87.4.818
Kirschbaum MUF, Gross LJ, Pearcy RW (1988) Observed and modelled stomatal responses to dynamic light environments in the shade plant Alocasia macrorrhiza. Plant Cell Environ 11:111–121
Ku SB, Edwards GE (1977) Oxygen inhibition of photosynthesis. Plant Physiol 59:986–990. doi:10.1007/BF00389372
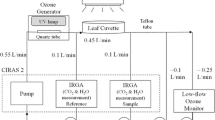
Küppers M, Häder DP (1999) Methodik der Photosyntheseforschung—Messung und Interpretation des CO2-Gasaustausches von intakten Blättern. In: Häder DP (ed) Photosynthese. Thieme, Stuttgart, pp 21–47
Küppers M, Pfiz M (2009) Role of photosynthetic induction for daily and annual carbon gains of leaves and plant canopies. In: Laisk A, Nedbal L, Govindjee (eds) Photosynthesis in silico. Springer, Netherlands, pp 417–440
Küppers M, Schneider H (1993) Leaf gas exchange of beech (Fagus sylvatica L.) seedlings in lightflecks: effects of fleck length and leaf temperature in leaves grown in deep and partial shade. Trees 7:160–168. doi:10.1007/BF00199617
Küppers M, Hall AE, Schulze ED (1982) Effects of day-to-day changes in root temperature on leaf conductance to water vapour and CO2 assimilation rates of Vigna unguiculata L. Walp. Oecologia (Berl) 52:116–120
Küppers M, Swan AG, Tompkins D et al (1987) A field portable system for the measurement of gas exchange of leaves under natural and controlled conditions: examples with field-grown Eucalyptus pauciflora Sieb. ex Spreng. ssp. pauciflora, E. behriana F. Muell. and Pinus radiata R. Don. Plant Cell Environ 10:425–435. doi:10.1111/1365-3040.ep11603690
Küppers M, Timm HC, Orth F et al (1996) Effects of light environment and successional status on lightfleck use by understory trees of temperate and tropical forests. Tree Physiol 16:69–80
Lange OL, Lösch R, Schulze ED, Kappen L (1971) Responses of stomata to changes in humidity. Planta 100:76–86. doi:10.1007/BF00386887
Lange OL, Schulze ED, Evenari M et al (1974) The temperature-related photosynthetic capacity of plants under desert conditions—I. Seasonal changes of the photosynthetic response to temperature. Oecologia 17:97–110. doi:10.1007/BF00346273
Leakey ADB, Press MC, Scholes JD (2003) High-temperature inhibition of photosynthesis is greater under sunflecks than uniform irradiance in a tropical rain forest tree seedling. Plant Cell Environ 26:1681–1690. doi:10.1046/j.1365-3040.2003.01086.x
Medlyn BE, Dreyer E, Ellsworth D et al (2002) Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Environ 25:1167–1179. doi:10.1046/j.1365-3040.2002.00891.x
Merilo E, Jõesaar I, Brosché M, Kollist H (2014) To open or to close: species-specific stomatal responses to simultaneously applied opposing environmental factors. New Phytol 202:499–508. doi:10.1111/nph.12667
Mott KA, Peak D (2010) Stomatal responses to humidity and temperature in darkness. Plant Cell Environ 33:1084–1090. doi:10.1111/j.1365-3040.2010.02129.x
Mott KA, Woodrow IE (1993) Effects of O2 and CO2 on nonsteady-state photosynthesis. Plant Physiol 102:859–866
Mott KA, Sibbernsen ED, Shope JC (2008) The role of the mesophyll in stomatal responses to light and CO2. Plant Cell Environ 31:1299–1306. doi:10.1111/j.1365-3040.2008.01845.x
Naumburg E, Ellsworth DS (2000) Photosynthetic sunfleck utilization potential of understory saplings growing under elevated CO2 in FACE. Oecologia 122:163–174. doi:10.1007/PL00008844
Naumburg E, Ellsworth DS (2002) Short-term light and leaf photosynthetic dynamics affect estimates of daily understory photosynthesis in four tree species. Tree Physiol 22:393–401
Ögren E, Sundin U (1996) Photosynthetic responses to variable light: a comparison of species from contrasting habitats. Oecologia 106:18–27
Ozturk I, Holst N, Ottosen CO (2012) Simulation of leaf photosynthesis of C3 plants under fluctuating light and different temperatures. Acta Physiol Plant 34:2319–2329. doi:10.1007/s11738-012-1033-8
Ozturk I, Ottosen CO, Ritz C (2013) The effect of temperature on photosynthetic induction under fluctuating light in Chrysanthemum morifolium. Acta Physiol Plant 35:1179–1188. doi:10.1007/s11738-012-1157-x
Pearcy RW, Chazdon RL, Gross LI, Mott KA (1994) Photosynthetic utilization of sunflecks: a temporally patchy resource on a time scale of seconds to minutes. In: Caldwell MM, Pearcy RW (eds) Exploitation of environmental heterogeneity by plants. Academic Press, San Diego, pp 175–208
Peguero-Pina JJ, Sisó S, Flexas J et al (2017) Cell-level anatomical characteristics explain high mesophyll conductance and photosynthetic capacity in sclerophyllous Mediterranean oaks. New Phytol. doi:10.1111/nph.14406
Pfitsch WA, Pearcy RW (1989) Daily carbon gain by Adenocaulon bicolor (Asteraceae), a redwood forest understory herb, in relation to its light environment. Oecologia 80:465–470
Radin JW, Lu Z, Percy RG, Zeiger E (1994) Genetic variability for stomatal conductance in Pima cotton and its relation to improvements of heat adaptation. Proc Natl Acad Sci USA 91:7217–7221. doi:10.1073/pnas.91.15.7217
Resco de Dios V, Gessler A, Ferrio JP et al (2016) Circadian rhythms have significant effects on leaf-to-canopy scale gas exchange under field conditions. Gigascience 5:43. doi:10.1186/s13742-016-0149-y
Roden JS, Pearcy RW (1993) Effect of leaf flutter on the light environment of poplars. Oecologia 93:201–207. doi:10.1007/BF00317672
Salvucci ME, Crafts-Brandner SJ (2004) Inhibition of photosynthesis by heat stress: the activation state of Rubisco as a limiting factor in photosynthesis. Physiol Plant 120:179–186. doi:10.1111/j.0031-9317.2004.0173.x
Sassenrath-Cole GF, Pearcy RW (1992) The role of ribulose-1,5-bisphosphate regeneration in the induction requirement of photosynthetic CO(2) exchange under transient light conditions. Plant Physiol 99:227–234. doi:10.1104/pp.99.1.227
Schulze ED, Küppers M (1979) Short-term and long-term effects of plant water deficits on stomatal response to humidity in Corylus avellana L. Planta 146:319–326. doi:10.1007/BF00387804
Schulze ED, Lange OL, Kappen L et al (1973) Stomatal responses to changes in temperature at increasing water stress. Planta 110:29–42. doi:10.1007/BF00386920
Singsaas EL, Sharkey TD (1998) The regulation of isoprene emission responses to rapid leaf temperature fluctuations. Plant Cell Environ 21:1181–1188. doi:10.1046/j.1365-3040.1998.00380.x
Stegemann J, Timm HC, Küppers M (1996) Light environment and photosynthesis of an understorey and a pioneer species from a premontane rainforest of Costa Rica. In: Ortiz R (ed) Revista Pensamiento Actual. Universidad de Costa Rica, San Jose, pp 61–68
Tenhunen JD, Lange OL, Braun M (1981) Midday stomatal closure in Mediterranean type sclerophylls under simulated habitat conditions in an environmental chamber—II. Effect of the complex of leaf temperature and air humidity on gas exchange of Arbutus unedo and Quercus ilex. Oecologia 50:5–11. doi:10.1007/BF00378788
Tenhunen JD, Lange OL, Gebel J et al (1984) Changes in photosynthetic capacity, carboxylation efficiency, and CO2 compensation point associated with midday stomatal closure and midday depression of net CO2 exchange of leaves of Quercus suber. Planta 162:193–203. doi:10.1007/BF00397440
Timm HC, Küppers M, Stegemann J (2004) Non-destructive analysis of architectural expansion and assimilate allocation in different tropical tree saplings: consequences of using steady-state and dynamic photosynthesis models. Ecotropica 10:101–121
Tinoco-Ojanguren C, Pearcy RW (1993a) Stomatal dynamics and its importance to carbon gain in two rainforest Piper species—II. Stomatal versus biochemical limitations during photosynthetic induction. Oecologia 94:395–402
Tinoco-Ojanguren C, Pearcy RW (1993b) Stomatal dynamics and its importance to carbon gain in two rainforest Piper species—I. VPD effects on the transient stomatal response to lightflecks. Oecologia 94:388–394. doi:10.1007/BF00317114
Valladares F, Allen MT, Pearcy RW (1997) Photosynthetic responses to dynamic light under field conditions in six tropical rainforest shrubs occurring along a light gradient. Oecologia 111:505–514. doi:10.1007/s004420050264
Vialet-Chabrand S, Dreyer E, Brendel O (2013) Performance of a new dynamic model for predicting diurnal time courses of stomatal conductance at the leaf level. Plant Cell Environ 36:1529–1546. doi:10.1111/pce.12086
Vico G, Manzoni S, Palmroth S, Katul G (2011) Effects of stomatal delays on the economics of leaf gas exchange under intermittent light regimes. New Phytol 192:640–652. doi:10.1111/j.1469-8137.2011.03847.x
von Caemmerer S, Evans JR (2015) Temperature responses of mesophyll conductance differ greatly between species. Plant Cell Environ 38:629–637. doi:10.1111/pce.12449
von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387. doi:10.1007/BF00384257
Wachendorf M, Küppers M (2017) The effect of initial stomatal opening on the dynamics of biochemical and overall photosynthetic induction. Trees 31:981–995. doi:10.1007/s00468-017-1522-x
Warren CR, Dreyer E (2006) Temperature response of photosynthesis and internal conductance to CO2: results from two independent approaches. J Exp Bot 57:3057–3067. doi:10.1093/jxb/erl067
Way DA, Pearcy RW (2012) Sunflecks in trees and forests: from photosynthetic physiology to global change biology. Tree Physiol 32:1066–1081. doi:10.1093/treephys/tps064
Yamori W (2016) Photosynthetic response to fluctuating environments and photoprotective strategies under abiotic stress. J Plant Res 129:379–395. doi:10.1007/s10265-016-0816-1
Yamori W, Noguchi K, Hanba YT, Terashima I (2006) Effects of internal conductance on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures. Plant Cell Physiol 47:1069–1080. doi:10.1093/pcp/pcj077
Yamori W, Masumoto C, Fukayama H, Makino A (2012) Rubisco activase is a key regulator of non-steady-state photosynthesis at any leaf temperature and to a lesser extent, of steady-state photosynthesis at high temperature. Plant J 71:871–880. doi:10.1111/j.1365-313X.2012.05041.x
Yamori W, Hikosaka K, Way DA (2014) Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosynth Res 119:101–117. doi:10.1007/s11120-013-9874-6
Young DR, Smith WK (1979) Influence of sunflecks on the temperature and water relations of two subalpine understory congeners. Oecologia 43:195–205
Zhang Q, Chen JW, Li BG, Cao KF (2009) Epiphytes and hemiepiphytes have slower photosynthetic response to lightflecks than terrestrial plants: evidence from ferns and figs. J Trop Ecol 25:465. doi:10.1017/S026646740900618X
Acknowledgements
We wish to thank Margaret Janke (Hohenheim) for correcting the English, Miko Kirschbaum (Palmerston North, New Zealand) for discussing parts of the early results and unknown reviewers for very helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by T. Grams.
Rights and permissions
About this article
Cite this article
Wachendorf, M., Küppers, M. Effects of leaf temperature on initial stomatal opening and their roles in overall and biochemical photosynthetic induction. Trees 31, 1667–1681 (2017). https://doi.org/10.1007/s00468-017-1577-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-017-1577-8