Abstract
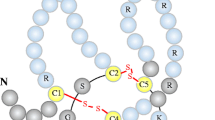
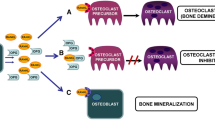
Canonical Wnt signaling activity contributes to physiological and adaptive bone mineralization and is an essential player in bone remodeling. Sclerostin is a prototypic soluble canonical Wnt signaling pathway inhibitor that is produced in osteocytes and blocks osteoblast differentiation and function. Therefore, sclerostin is a potent inhibitor of bone formation and mineralization. Accordingly, rodent sclerostin-deficiency models exhibit a strong bone phenotype. Moreover, blocking sclerostin represents a promising treatment perspective against osteoporosis. Beyond the bone field novel data definitely associate Wnt signaling in general and sclerostin in particular with ectopic extraosseous mineralization processes, as is evident in cardiovascular calcification or calciphylaxis. Uremia is characterized by parallel occurrence of disordered bone mineralization and accelerated cardiovascular calcification (chronic kidney disease – mineral and bone disorder, CKD-MBD), linking skeletal and cardiovascular disease—the so-called bone-vascular calcification paradox. In consequence, sclerostin may qualify as an emerging player in CKD-MBD. We present a stepwise review approach regarding the rapidly evolving field sclerostin participation in CKD-MBD. Starting from data originating in the classical bone field we look separately at three major areas of CKD-MBD: disturbed mineral metabolism, renal osteodystrophy, and uremic cardiovascular disease. Our review is intended to help the nephrologist revise the potential importance of sclerostin in CKD by focusing on how sclerostin research is gradually evolving from the classical osteoporosis niche into the area of CKD-MBD. In particular, we integrate the limited amount of available data in the context of pediatric nephrology.





Similar content being viewed by others
References
Kestler HA, Kuhl M (2008) From individual Wnt pathways towards a Wnt signalling network. Philos Trans R Soc Lond B Biol Sci 363:1333–1347
Kim W, Kim M, Jho EH (2013) Wnt/beta-catenin signalling: from plasma membrane to nucleus. Biochem J 450:9–21
Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ (2012) Update on Wnt signaling in bone cell biology and bone disease. Gene 492:1–18
Kubota T, Michigami T, Ozono K (2010) Wnt signaling in bone. Clin Pediatr Endocrinol 19:49–56
Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP (2007) LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 315:1278–1282
Dejana E (2010) The role of Wnt signaling in physiological and pathological angiogenesis. Circ Res 107:943–952
Johnson ML, Kamel MA (2007) The Wnt signaling pathway and bone metabolism. Curr Opin Rheumatol 19:376–382
Rajamannan NM (2011) The role of Lrp5/6 in cardiac valve disease: LDL-density-pressure theory. J Cell Biochem 112:2222–2229
Vervloet MG, Massy ZA, Brandenburg VM, Mazzaferro S, Cozzolino M, Urena-Torres P, Bover J, Goldsmith D (2014) Bone: a new endocrine organ at the heart of chronic kidney disease and mineral and bone disorders. Lancet Diabetes Endocrinol 2:427–436
Cozzolino M, Urena-Torres P, Vervloet MG, Brandenburg V, Bover J, Goldsmith D, Larsson TE, Massy ZA, Mazzaferro S (2014) Is chronic kidney disease-mineral bone disorder (CKD-MBD) really a syndrome? Nephrol Dial Transplant 29:1815–1820
Ke HZ, Richards WG, Li X, Ominsky MS (2012) Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev 33:747–783
Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA (2003) Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22:6267–6276
Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J (2005) Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J 19:1842–1844
Capulli M, Paone R, Rucci N (2014) Osteoblast and osteocyte: games without frontiers. Arch Biochem Biophys 561:3–12
Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ (2008) Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci U S A 105:20764–20769
Baron R, Rawadi G (2007) Wnt signaling and the regulation of bone mass. Curr Osteoporos Rep 5:73–80
Choi HY, Dieckmann M, Herz J, Niemeier A (2009) Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS One 4:e7930
Glass DA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8:751–764
Schmitz Y, Rateitschak K, Wolkenhauer O (2013) Analysing the impact of nucleo-cytoplasmic shuttling of beta-catenin and its antagonists APC, Axin and GSK3 on Wnt/beta-catenin signalling. Cell Signal 25:2210–2221
Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP (2002) High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med 346:1513–1521
Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, Reddy PS, Bodine PV, Robinson JA, Bhat B, Marzolf J, Moran RA, Bex F (2003) High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res 18:960–974
Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van Hul W (2002) Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet 39:91–97
Morvan F, Boulukos K, Clement-Lacroix P, Roman RS, Suc-Royer I, Vayssiere B, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G (2006) Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res 21:934–945
Robinson MK, Caminis J, Brunkow ME (2013) Sclerostin: how human mutations have helped reveal a new target for the treatment of osteoporosis. Drug Discov Today 18:637–643
Kramer I, Loots GG, Studer A, Keller H, Kneissel M (2010) Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J Bone Miner Res 25:178–189
Yao GQ, Wu JJ, Troiano N, Insogna K (2011) Targeted overexpression of Dkk1 in osteoblasts reduces bone mass but does not impair the anabolic response to intermittent PTH treatment in mice. J Bone Miner Metab 29:141–148
Ryan ZC, Ketha H, McNulty MS, McGee-Lawrence M, Craig TA, Grande JP, Westendorf JJ, Singh RJ, Kumar R (2013) Sclerostin alters serum vitamin D metabolite and fibroblast growth factor 23 concentrations and the urinary excretion of calcium. Proc Natl Acad Sci U S A 110:6199–6204
Register TC, Hruska KA, Divers J, Bowden DW, Palmer ND, Carr JJ, Wagenknecht LE, Hightower RC, Xu J, Smith SC, Dietzen DJ, Langefeld CD, Freedman BI (2014) Sclerostin is positively associated with bone mineral density in men and women and negatively associated with carotid calcified atherosclerotic plaque in men from the African American-Diabetes Heart Study. J Clin Endocrinol Metab 99:315–321
Dovjak P, Dorfer S, Foger-Samwald U, Kudlacek S, Marculescu R, Pietschmann P (2014) Serum levels of sclerostin and dickkopf-1: effects of age, gender and fracture status. Gerontology 60:493–501
Viapiana O, Fracassi E, Troplini S, Idolazzi L, Rossini M, Adami S, Gatti D (2013) Sclerostin and DKK1 in primary hyperparathyroidism. Calcif Tissue Int 92:324–329
Amrein K, Amrein S, Drexler C, Dimai HP, Dobnig H, Pfeifer K, Tomaschitz A, Pieber TR, Fahrleitner-Pammer A (2012) Sclerostin and its association with physical activity, age, gender, body composition, and bone mineral content in healthy adults. J Clin Endocrinol Metab 97:148–154
Bonewald LF (2011) The amazing osteocyte. J Bone Miner Res 26:229–238
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–192
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, Libanati C, Bone HG (2014) Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 370:412–420
van Lierop AH, Hamdy NA, Hamersma H, van Bezooijen RL, Power J, Loveridge N, Papapoulos SE (2011) Patients with sclerosteosis and disease carriers: human models of the effect of sclerostin on bone turnover. J Bone Miner Res 26:2804–2811
Evenepoel P, D’Haese P, Brandenburg V (2014) Romosozumab in postmenopausal women with osteopenia. N Engl J Med 370:1664
Nakamura T, Nakamura T, Matsumoto K (2008) The functions and possible significance of Kremen as the gatekeeper of Wnt signalling in development and pathology. J Cell Mol Med 12:391–408
Gifre L, Ruiz-Gaspa S, Monegal A, Nomdedeu B, Filella X, Guanabens N, Peris P (2013) Effect of glucocorticoid treatment on Wnt signalling antagonists (sclerostin and Dkk-1) and their relationship with bone turnover. Bone 57:272–276
Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, Niehrs C, Izpisua Belmonte JC, Westphal H (2001) Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell 1:423–434
Hampson G, Edwards S, Conroy S, Blake GM, Fogelman I, Frost ML (2013) The relationship between inhibitors of the Wnt signalling pathway (Dickkopf-1(DKK1) and sclerostin), bone mineral density, vascular calcification and arterial stiffness in post-menopausal women. Bone 56:42–47
Vivante A, Mark-Danieli M, Davidovits M, Harari-Steinberg O, Omer D, Gnatek Y, Cleper R, Landau D, Kovalski Y, Weissman I, Eisenstein I, Soudack M, Wolf HR, Issler N, Lotan D, Anikster Y, Dekel B (2013) Renal hypodysplasia associates with a WNT4 variant that causes aberrant canonical WNT signaling. J Am Soc Nephrol 24:550–558
He W, Kang YS, Dai C, Liu Y (2011) Blockade of Wnt/beta-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol 22:90–103
Fang Y, Ginsberg C, Seifert M, Agapova O, Sugatani T, Register TC, Freedman BI, Monier-Faugere MC, Malluche H, Hruska KA (2014) CKD-induced wingless/integration1 inhibitors and phosphorus cause the CKD-mineral and bone disorder. J Am Soc Nephrol 25:1760–1773
Floege J (2014) Antagonism of canonical Wnt/beta-catenin signaling: taking RAS blockade to the next level? J Am Soc Nephrol 26:3–5
Cannata-Andia JB, Roman-Garcia P, Hruska K (2011) The connections between vascular calcification and bone health. Nephrol Dial Transplant 26:3429–3436
Brandenburg VM, Kramann R, Koos R, Kruger T, Schurgers L, Muhlenbruch G, Hubner S, Gladziwa U, Drechsler C, Ketteler M (2013) Relationship between sclerostin and cardiovascular calcification in hemodialysis patients: a cross-sectional study. BMC Nephrol 14:219
Cejka D, Marculescu R, Kozakowski N, Plischke M, Reiter T, Gessl A, Haas M (2014) Renal elimination of sclerostin increases with declining kidney function. J Clin Endocrinol Metab 99:248–255
Bonani M, Rodriguez D, Fehr T, Mohebbi N, Brockmann J, Blum M, Graf N, Frey D, Wuthrich RP (2014) Sclerostin blood levels before and after kidney transplantation. Kidney Blood Press Res 39:230–239
Sabbagh Y, Graciolli FG, O’Brien S, Tang W, dos Reis LM, Ryan S, Phillips L, Boulanger J, Song W, Bracken C, Liu S, Ledbetter S, Dechow P, Canziani ME, Carvalho AB, Jorgetti V, Moyses RM, Schiavi SC (2012) Repression of osteocyte Wnt/beta-catenin signaling is an early event in the progression of renal osteodystrophy. J Bone Miner Res 27:1757–1772
Viaene L, Behets GJ, Claes K, Meijers B, Blocki F, Brandenburg V, Evenepoel P, D’Haese PC (2013) Sclerostin: another bone-related protein related to all-cause mortality in haemodialysis? Nephrol Dial Transplant 28:3024–3030
Drechsler C, Evenepoel P, Vervloet MG, Wanner C, Ketteler M, Marx N, Floege J, Dekker FW, Brandenburg VM (2014) High levels of circulating sclerostin are associated with better cardiovascular survival in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transplant. doi:10.1093/ndt/gfu301
Kanbay M, Siriopol D, Saglam M, Gulcan KY, Gok M, Cetinkaya H, Karaman M, Umut UH, Oguz Y, Sari S, Eyileten T, Goldsmith D, Vural A, Veisa G, Covic A, Ilker YM (2014) Serum sclerostin and adverse outcomes in non-dialyzed chronic kidney disease patients. J Clin Endocrinol Metab 99:E1854–E1861
Bellido T, Saini V, Pajevic PD (2013) Effects of PTH on osteocyte function. Bone 54:250–257
Keller H, Kneissel M (2005) SOST is a target gene for PTH in bone. Bone 37:148–158
Durosier C, van Lierop A, Ferrari S, Chevalley T, Papapoulos S, Rizzoli R (2013) Association of circulating sclerostin with bone mineral mass, microstructure, and turnover biochemical markers in healthy elderly men and women. J Clin Endocrinol Metab 98:3873–3883
Li C, Xing Q, Yu B, Xie H, Wang W, Shi C, Crane JL, Cao X, Wan M (2013) Disruption of LRP6 in osteoblasts blunts the bone anabolic activity of PTH. J Bone Miner Res 28:2094–2108
Cannata-Andia JB, Rodriguez GM, Gomez AC (2013) Osteoporosis and adynamic bone in chronic kidney disease. J Nephrol 26:73–80
Cejka D, Herberth J, Branscum AJ, Fardo DW, Monier-Faugere MC, Diarra D, Haas M, Malluche HH (2011) Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin J Am Soc Nephrol 6:877–882
Ferreira JC, Ferrari GO, Neves KR, Cavallari RT, Dominguez WV, dos Reis LM, Graciolli FG, Oliveira EC, Liu S, Sabbagh Y, Jorgetti V, Schiavi S, Moyses RM (2013) Effects of dietary phosphate on adynamic bone disease in rats with chronic kidney disease–role of sclerostin? PLoS One 8:e79721
Marinou K, Christodoulides C, Antoniades C, Koutsilieris M (2012) Wnt signaling in cardiovascular physiology. Trends Endocrinol Metab 23:628–636
Sarzani R, Salvi F, Bordicchia M, Guerra F, Battistoni I, Pagliariccio G, Carbonari L, Dessi-Fulgheri P, Rappelli A (2011) Carotid artery atherosclerosis in hypertensive patients with a functional LDL receptor-related protein 6 gene variant. Nutr Metab Cardiovasc Dis 21:150–156
Cozzolino M, Brandenburg V (2010) Warfarin: to use or not to use in chronic kidney disease patients? J Nephrol 23:648–652
Kruger T, Oelenberg S, Kaesler N, Schurgers LJ, van de Sandt AM, Boor P, Schlieper G, Brandenburg VM, Fekete BC, Veulemans V, Ketteler M, Vermeer C, Jahnen-Dechent W, Floege J, Westenfeld R (2013) Warfarin induces cardiovascular damage in mice. Arterioscler Thromb Vasc Biol 33:2618–2624
Beazley KE, Deasey S, Lima F, Nurminskaya MV (2012) Transglutaminase 2-mediated activation of beta-catenin signaling has a critical role in warfarin-induced vascular calcification. Arterioscler Thromb Vasc Biol 32:123–130
Zhu D, Mackenzie NC, Millan JL, Farquharson C, MacRae VE (2011) The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS One 6:e19595
Koos R, Brandenburg V, Mahnken AH, Schneider R, Dohmen G, Autschbach R, Marx N, Kramann R (2013) Sclerostin as a potential novel biomarker for aortic valve calcification: an in-vivo and ex-vivo study. J Heart Valve Dis 22:317–325
Kramann R, Brandenburg VM, Schurgers LJ, Ketteler M, Westphal S, Leisten I, Bovi M, Jahnen-Dechent W, Knuchel R, Floege J, Schneider RK (2013) Novel insights into osteogenesis and matrix remodelling associated with calcific uraemic arteriolopathy. Nephrol Dial Transplant 28:856–868
Claes KJ, Viaene L, Heye S, Meijers B, D’Haese P, Evenepoel P (2013) Sclerostin: another vascular calcification inhibitor? J Clin Endocrinol Metab 98:3221–3228
Modder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Riggs BL, Melton LJ III, Khosla S (2011) Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res 26:373–379
Fischer DC, Mischek A, Wolf S, Rahn A, Salweski B, Kundt G, Haffner D (2012) Paediatric reference values for the C-terminal fragment of fibroblast-growth factor-23, sclerostin, bone-specific alkaline phosphatase and isoform 5b of tartrate-resistant acid phosphatase. Ann Clin Biochem 49:546–553
Kirmani S, Amin S, McCready LK, Atkinson EJ, Melton LJ III, Muller R, Khosla S (2012) Sclerostin levels during growth in children. Osteoporos Int 23:1123–1130
Drake MT, Srinivasan B, Modder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S (2010) Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab 95:5056–5062
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brandenburg, V.M., D’Haese, P., Deck, A. et al. From skeletal to cardiovascular disease in 12 steps—the evolution of sclerostin as a major player in CKD-MBD. Pediatr Nephrol 31, 195–206 (2016). https://doi.org/10.1007/s00467-015-3069-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-015-3069-7