Abstract
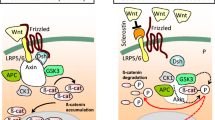
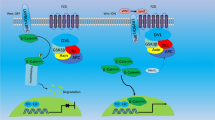
In the course of chronic kidney disease (CKD), alterations in the bone-vascular axis augment the risk of bone loss, fractures, vascular and soft tissue calcification, left ventricular hypertrophy, renal and myocardial fibrosis, which markedly increase morbidity and mortality rates. A major challenge to improve skeletal and cardiovascular outcomes in CKD patients requires a better understanding of the increasing complex interactions among the main modulators of the bone-vascular axis. Serum parathyroid hormone (PTH), phosphorus (P), calcium (Ca), fibroblast growth factor 23 (FGF23), calcidiol, calcitriol and Klotho are involved in this axis interact with RANK/RANKL/OPG system and the Wnt/β-catenin pathway. The RANK/RANKL/OPG system controls bone remodeling by inducing osteoblast synthesis of RANKL and downregulating OPG production and it is also implicated in vascular calcification. The complexity of this system has recently increased due the discovery of LGR4, a novel RANKL receptor involved in bone formation, but possibly also in vascular calcification. The Wnt/β-catenin pathway plays a key role in bone formation: when this pathway is activated, bone is formed, but when it is inhibited, bone formation is stopped. In the progression of CKD, a downregulation of the Wnt/β-catenin pathway has been described which occurs mainly through the not coincident elevations of sclerostin, Dickkopf1 (Dkk1) and the secreted Frizzled Related Proteins (sFRPs). This review analyzes the interactions of PTH, P, Ca, FGF23, calcidiol, calcitriol and Klotho with the RANKL/RANKL/OPG system and the Wnt/β-catenin, pathway and their implications in bone and cardiovascular disorders in CKD.


Similar content being viewed by others
References
Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V (2004) Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab 89(9):4246–4253
Naves M, Rodriguez-Garcia M, Diaz-Lopez JB, Gomez-Alonso C, Cannata-Andia JB (2008) Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos Int 19(8):1161–1166
Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O’Donnell CJ, Wilson PW (2001) Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int 68(5):271–276
Frye MA, Melton LJ 3rd, Bryant SC, Fitzpatrick LA, Wahner HW, Schwartz RS et al (1992) Osteoporosis and calcification of the aorta. Bone Miner 19(2):185–194
Cannata-Andia JB, Rodriguez-Garcia M, Carrillo-Lopez N, Naves-Diaz M, Diaz-Lopez B (2006) Vascular calcifications: pathogenesis, management, and impact on clinical outcomes. J Am Soc Nephrol 17(12 Suppl 3):S267–S273
Adragao T, Herberth J, Monier-Faugere MC, Branscum AJ, Ferreira A, Frazao JM et al (2009) Low bone volume–a risk factor for coronary calcifications in hemodialysis patients. Clin J Am Soc Nephrol 4(2):450–455
Coen G, Ballanti P, Mantella D, Manni M, Lippi B, Pierantozzi A et al (2009) Bone turnover, osteopenia and vascular calcifications in hemodialysis patients. A histomorphometric and multislice CT study. Am J Nephrol 29(3):145–152
Chen H, Han X, Cui Y, Ye Y, Purrunsing Y, Wang N (2018) Parathyroid hormone fragments: new targets for the diagnosis and treatment of chronic kidney disease-mineral and bone disorder. Biomed Res Int 2018:9619253
Almaden Y, Canalejo A, Hernandez A, Ballesteros E, Garcia-Navarro S, Torres A et al (1996) Direct effect of phosphorus on PTH secretion from whole rat parathyroid glands in vitro. J Bone Miner Res 11(7):970–976
Almaden Y, Hernandez A, Torregrosa V, Canalejo A, Sabate L, Fernandez Cruz L et al (1998) High phosphate level directly stimulates parathyroid hormone secretion and synthesis by human parathyroid tissue in vitro. J Am Soc Nephrol 9(10):1845–1852
Kilav R, Silver J, Naveh-Many T (1995) Parathyroid hormone gene expression in hypophosphatemic rats. J Clin Invest 96(1):327–333
Slatopolsky E, Brown A, Dusso A (1999) Pathogenesis of secondary hyperparathyroidism. Kidney Int Suppl 73:S14–S19
Neves KR, Graciolli FG, dos Reis LM, Graciolli RG, Neves CL, Magalhaes AO et al (2007) Vascular calcification: contribution of parathyroid hormone in renal failure. Kidney Int 71(12):1262–1270
Shao JS, Cheng SL, Charlton-Kachigian N, Loewy AP, Towler DA (2003) Teriparatide (human parathyroid hormone (1–34)) inhibits osteogenic vascular calcification in diabetic low density lipoprotein receptor-deficient mice. J Biol Chem 278(50):50195–50202
Vattikuti R, Towler DA (2004) Osteogenic regulation of vascular calcification: an early perspective. Am J Physiol 286(5):E686–E696
Graciolli FG, Neves KR, dos Reis LM, Graciolli RG, Noronha IL, Moyses RM et al (2009) Phosphorus overload and PTH induce aortic expression of Runx2 in experimental uraemia. Nephrol Dial Transpl 24(5):1416–1421
Carrillo-Lopez N, Panizo S, Alonso-Montes C, Martinez-Arias L, Avello N, Sosa P et al (2019) High-serum phosphate and parathyroid hormone distinctly regulate bone loss and vascular calcification in experimental chronic kidney disease. Nephrol Dial Transpl 34(6):934–941
Hu MC, Shi M, Zhang J, Addo T, Cho HJ, Barker SL et al (2016) Renal production, uptake, and handling of circulating alpha klotho. J Am Soc Nephrol 27(1):79–90
Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG (2005) The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310(5747):490–493
Alexander RT, Woudenberg-Vrenken TE, Buurman J, Dijkman H, van der Eerden BC, van Leeuwen JP et al (2009) Klotho prevents renal calcium loss. J Am Soc Nephrol 20(11):2371–2379
Huang JC, Sakata T, Pfleger LL, Bencsik M, Halloran BP, Bikle DD et al (2004) PTH differentially regulates expression of RANKL and OPG. J Bone Miner Res 19(2):235–244
Fu Q, Jilka RL, Manolagas SC, O’Brien CA (2002) Parathyroid hormone stimulates receptor activator of NFkappa B ligand and inhibits osteoprotegerin expression via protein kinase A activation of cAMP-response element-binding protein. J Biol Chem 277(50):48868–48875
Ben-awadh AN, Delgado-Calle J, Tu X, Kuhlenschmidt K, Allen MR, Plotkin LI et al (2014) Parathyroid hormone receptor signaling induces bone resorption in the adult skeleton by directly regulating the RANKL gene in osteocytes. Endocrinology 155(8):2797–2809
Lee SK, Lorenzo JA (2002) Regulation of receptor activator of nuclear factor-kappa B ligand and osteoprotegerin mRNA expression by parathyroid hormone is predominantly mediated by the protein kinase a pathway in murine bone marrow cultures. Bone 31(1):252–259
Boyce BF, Xing L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 473(2):139–146
Panizo S, Cardus A, Encinas M, Parisi E, Valcheva P, Lopez-Ongil S et al (2009) RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ Res 104(9):1041–1048
Kawakami R, Nakagami H, Noma T, Ohmori K, Kohno M, Morishita R (2016) RANKL system in vascular and valve calcification with aging. Inflamm Regen 36:10
Boyce BF, Xing L (2007) Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther 9(Suppl 1):S1
Nelson CA, Warren JT, Wang MW, Teitelbaum SL, Fremont DH (2012) RANKL employs distinct binding modes to engage RANK and the osteoprotegerin decoy receptor. Structure 20(11):1971–1982
Collin-Osdoby P, Rothe L, Anderson F, Nelson M, Maloney W, Osdoby P (2001) Receptor activator of NF-kappa B and osteoprotegerin expression by human microvascular endothelial cells, regulation by inflammatory cytokines, and role in human osteoclastogenesis. J Biol Chem 276(23):20659–20672
Sattler AM, Schoppet M, Schaefer JR, Hofbauer LC (2004) Novel aspects on RANK ligand and osteoprotegerin in osteoporosis and vascular disease. Calcif Tissue Int 74(1):103–106
Ono T, Hayashi M, Sasaki F, Nakashima T (2020) RANKL biology: bone metabolism, the immune system, and beyond. Inflamm Regen 40:2
Hanada R, Leibbrandt A, Hanada T, Kitaoka S, Furuyashiki T, Fujihara H et al (2009) Central control of fever and female body temperature by RANKL/RANK. Nature 462(7272):505–509
Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA et al (2000) The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103(1):41–50
Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T et al (1999) RANK is essential for osteoclast and lymph node development. Genes Dev 13(18):2412–2424
Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER et al (1997) A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390(6656):175–179
Green EA, Flavell RA (1999) TRANCE-RANK, a new signal pathway involved in lymphocyte development and T cell activation. J Exp Med 189(7):1017–1020
Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E et al (1999) Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA 96(7):3540–3545
Myers DE, Collier FM, Minkin C, Wang H, Holloway WR, Malakellis M et al (1999) Expression of functional RANK on mature rat and human osteoclasts. FEBS Lett 463(3):295–300
Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Yano K et al (1998) RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem Biophys Res Commun 253(2):395–400
Kong YY, Boyle WJ, Penninger JM (2000) Osteoprotegerin ligand: a regulator of immune responses and bone physiology. Immunol Today 21(10):495–502
Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S et al (1999) Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402(6759):304–309
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T et al (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93(2):165–176
Jimi E, Akiyama S, Tsurukai T, Okahashi N, Kobayashi K, Udagawa N et al (1999) Osteoclast differentiation factor acts as a multifunctional regulator in murine osteoclast differentiation and function. J Immunol 163(1):434–442
Wong BR, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H et al (1999) TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell 4(6):1041–1049
Graham TR, Odero-Marah VA, Chung LW, Agrawal KC, Davis R, Abdel-Mageed AB (2009) PI3K/Akt-dependent transcriptional regulation and activation of BMP-2-Smad signaling by NF-kappaB in metastatic prostate cancer cells. Prostate 69(2):168–180
Yun TJ, Chaudhary PM, Shu GL, Frazer JK, Ewings MK, Schwartz SM et al (1998) OPG/FDCR-1, a TNF receptor family member, is expressed in lymphoid cells and is up-regulated by ligating CD40. J Immunol 161(11):6113–6121
Schoppet M, Preissner KT, Hofbauer LC (2002) RANK ligand and osteoprotegerin: paracrine regulators of bone metabolism and vascular function. Arterioscler Thromb Vasc Biol 22(4):549–553
Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Spelsberg TC, Riggs BL (1999) Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology 140(9):4367–4370
Saika M, Inoue D, Kido S, Matsumoto T (2001) 17beta-estradiol stimulates expression of osteoprotegerin by a mouse stromal cell line, ST-2, via estrogen receptor-alpha. Endocrinology 142(6):2205–2212
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R et al (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89(2):309–319
Takai H, Kanematsu M, Yano K, Tsuda E, Higashio K, Ikeda K et al (1998) Transforming growth factor-beta stimulates the production of osteoprotegerin/osteoclastogenesis inhibitory factor by bone marrow stromal cells. J Biol Chem 273(42):27091–27096
Vidal ON, Sjogren K, Eriksson BI, Ljunggren O, Ohlsson C (1998) Osteoprotegerin mRNA is increased by interleukin-1 alpha in the human osteosarcoma cell line MG-63 and in human osteoblast-like cells. Biochem Biophys Res Commun 248(3):696–700
Makiishi-Shimobayashi C, Tsujimura T, Iwasaki T, Yamada N, Sugihara A, Okamura H et al (2001) Interleukin-18 up-regulates osteoprotegerin expression in stromal/osteoblastic cells. Biochem Biophys Res Commun 281(2):361–366
Brandstrom H, Bjorkman T, Ljunggren O (2001) Regulation of osteoprotegerin secretion from primary cultures of human bone marrow stromal cells. Biochem Biophys Res Commun 280(3):831–835
Brandstrom H, Jonsson KB, Ohlsson C, Vidal O, Ljunghall S, Ljunggren O (1998) Regulation of osteoprotegerin mRNA levels by prostaglandin E2 in human bone marrow stroma cells. Biochem Biophys Res Commun 247(2):338–341
Hofbauer LC, Gori F, Riggs BL, Lacey DL, Dunstan CR, Spelsberg TC et al (1999) Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis1. Endocrinology 140(10):4382–4389
Hofbauer LC, Shui C, Riggs BL, Dunstan CR, Spelsberg TC, O’Brien T et al (2001) Effects of immunosuppressants on receptor activator of NF-kappaB ligand and osteoprotegerin production by human osteoblastic and coronary artery smooth muscle cells. Biochem Biophys Res Commun 280(1):334–339
Nakagawa N, Yasuda H, Yano K, Mochizuki S, Kobayashi N, Fujimoto H et al (1999) Basic fibroblast growth factor induces osteoclast formation by reciprocally regulating the production of osteoclast differentiation factor and osteoclastogenesis inhibitory factor in mouse osteoblastic cells. Biochem Biophys Res Commun 265(1):158–163
Onyia JE, Miles RR, Yang X, Halladay DL, Hale J, Glasebrook A et al (2000) In vivo demonstration that human parathyroid hormone 1–38 inhibits the expression of osteoprotegerin in bone with the kinetics of an immediate early gene. J Bone Miner Res 15(5):863–871
Nitta K, Akiba T, Suzuki K, Uchida K, Watanabe R, Majima K et al (2004) Effects of cyclic intermittent etidronate therapy on coronary artery calcification in patients receiving long-term hemodialysis. Am J Kidney Dis 44(4):680–688
Yamada S, Leaf EM, Chia JJ, Cox TC, Speer MY, Giachelli CM (2018) PiT-2, a type III sodium-dependent phosphate transporter, protects against vascular calcification in mice with chronic kidney disease fed a high-phosphate diet. Kidney Int 94(4):716–727
Pulsatelli L, Dolzani P, Silvestri T, Caraceni P, Facchini A, Ravaglia G et al (2004) Soluble receptor activator of nuclear factor- kappaB Ligand (sRANKL)/osteoprotegerin balance in ageing and age-associated diseases. Biogerontology 5(2):119–127
Jung K, Lein M, Hösslin K, Grosse A, Roth S, Possinger K et al (2002) Osteoprotegerin and receptor activator of nuclear factor-kappaB ligand (RANKL) in the serum of healthy adults. Int J Biol Markers 17(3):177–181
Khosla S, Arrighi HM, Melton LJ 3rd, Atkinson EJ, O’Fallon WM, Dunstan C et al (2002) Correlates of osteoprotegerin levels in women and men. Osteoporos Int 13(5):394–399
Rogers A, Saleh G, Hannon RA, Greenfield D, Eastell R (2002) Circulating estradiol and osteoprotegerin as determinants of bone turnover and bone density in postmenopausal women. J Clin Endocrinol Metab 87(10):4470–4475
Szulc P, Hofbauer LC, Heufelder AE, Roth S, Delmas PD (2001) Osteoprotegerin serum levels in men: correlation with age, estrogen, and testosterone status. J Clin Endocrinol Metab 86(7):3162–3165
Helas S, Goettsch C, Schoppet M, Zeitz U, Hempel U, Morawietz H et al (2009) Inhibition of receptor activator of NF-kappaB ligand by denosumab attenuates vascular calcium deposition in mice. Am J Pathol 175(2):473–478
Luo J, Yang Z, Ma Y, Yue Z, Lin H, Qu G et al (2016) LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat Med 22(5):539–546
de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H et al (2011) Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476(7360):293–297
Luo J, Zhou W, Zhou X, Li D, Weng J, Yi Z et al (2009) Regulation of bone formation and remodeling by G-protein-coupled receptor 48. Development 136(16):2747–2756
Styrkarsdottir U, Thorleifsson G, Sulem P, Gudbjartsson DF, Sigurdsson A, Jonasdottir A et al (2013) Nonsense mutation in the LGR4 gene is associated with several human diseases and other traits. Nature 497(7450):517–520
Carrillo-Lopez N, Martinez-Arias L, Alonso-Montes C, Martin-Carro B, Martin-Virgala J, Ruiz-Ortega M, et al. (2020) The receptor activator of nuclear factor κΒ ligand receptor leucine-rich repeat-containing G-protein-coupled receptor 4 contributes to parathyroid hormone-induced vascular calcification. Nephrol Dial Transpl. https://doi.org/10.1093/ndt/gfaa290
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S et al (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95(7):3597–3602
Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C et al (1998) Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 12(9):1260–1268
Malyankar UM, Scatena M, Suchland KL, Yun TJ, Clark EA, Giachelli CM (2000) Osteoprotegerin is an alpha vbeta 3-induced, NF-kappa B-dependent survival factor for endothelial cells. J Biol Chem 275(28):20959–20962
Min H, Morony S, Sarosi I, Dunstan CR, Capparelli C, Scully S et al (2000) Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med 192(4):463–474
Boukhris R, Becker KL (1972) Calcification of the aorta and osteoporosis. A roentgenographic study. JAMA 219(10):1307–1311
Hak AE, Pols HA, van Hemert AM, Hofman A, Witteman JC (2000) Progression of aortic calcification is associated with metacarpal bone loss during menopause: a population-based longitudinal study. Arterioscler Thromb Vasc Biol 20(8):1926–1931
Kado DM, Browner WS, Blackwell T, Gore R, Cummings SR (2000) Rate of bone loss is associated with mortality in older women: a prospective study. J Bone Miner Res 15(10):1974–1980
Price PA, June HH, Buckley JR, Williamson MK (2001) Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler Thromb Vasc Biol 21(10):1610–1616
Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ et al (2001) Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol 21(12):1998–2003
Weiss RM, Lund DD, Chu Y, Brooks RM, Zimmerman KA, El Accaoui R et al (2013) Osteoprotegerin inhibits aortic valve calcification and preserves valve function in hypercholesterolemic mice. PLoS ONE 8(6):e65201
Carmon KS, Gong X, Lin Q, Thomas A, Liu Q (2011) R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci USA 108(28):11452–11457
Xu R, Zhang ZZ, Chen LJ, Yu HM, Guo SJ, Xu YL et al (2016) Ascending aortic adventitial remodeling and fibrosis are ameliorated with Apelin-13 in rats after TAC via suppression of the miRNA-122 and LGR4-β-catenin signaling. Peptides 86:85–94
Hofbauer LC, Schoppet M (2004) Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA 292(4):490–495
Osorio A, Ortega E, Torres JM, Sanchez P, Ruiz-Requena E (2013) Biochemical markers of vascular calcification in elderly hemodialysis patients. Mol Cell Biochem 374(1–2):21–27
Nitta K, Akiba T, Uchida K, Otsubo S, Takei T, Yumura W et al (2004) Serum osteoprotegerin levels and the extent of vascular calcification in haemodialysis patients. Nephrol Dial Transpl 19(7):1886–1889
Samelson EJ, Miller PD, Christiansen C, Daizadeh NS, Grazette L, Anthony MS et al (2014) RANKL inhibition with denosumab does not influence 3-year progression of aortic calcification or incidence of adverse cardiovascular events in postmenopausal women with osteoporosis and high cardiovascular risk. J Bone Miner Res 29(2):450–457
Komiya Y, Habas R (2008) Wnt signal transduction pathways. Organogenesis 4(2):68–75
MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17(1):9–26
Westendorf JJ, Kahler RA, Schroeder TM (2004) Wnt signaling in osteoblasts and bone diseases. Gene 341:19–39
Rawadi G, Roman-Roman S (2005) Wnt signalling pathway: a new target for the treatment of osteoporosis. Expert Opin Ther Targets 9(5):1063–1077
Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD et al (2005) Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA 102(9):3324–3329
Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS et al (2005) Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem 280(39):33132–33140
Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B et al (2003) The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 349(26):2483–2494
Qiang YW, Barlogie B, Rudikoff S, Shaughnessy JD Jr (2008) Dkk1-induced inhibition of Wnt signaling in osteoblast differentiation is an underlying mechanism of bone loss in multiple myeloma. Bone 42(4):669–680
Bodine PV, Zhao W, Kharode YP, Bex FJ, Lambert AJ, Goad MB et al (2004) The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol 18(5):1222–1237
Lu W, Kim KA, Liu J, Abo A, Feng X, Cao X et al (2008) R-spondin1 synergizes with Wnt3A in inducing osteoblast differentiation and osteoprotegerin expression. FEBS Lett 582(5):643–650
Kulkarni NH, Halladay DL, Miles RR, Gilbert LM, Frolik CA, Galvin RJ et al (2005) Effects of parathyroid hormone on Wnt signaling pathway in bone. J Cell Biochem 95(6):1178–1190
Silva BC, Bilezikian JP (2015) Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol 22:41–50
Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA et al (2005) Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 146(11):4577–4583
Keller H, Kneissel M (2005) SOST is a target gene for PTH in bone. Bone 37(2):148–158
Moe SM, Chen NX, Newman CL, Organ JM, Kneissel M, Kramer I et al (2015) Anti-sclerostin antibody treatment in a rat model of progressive renal osteodystrophy. J Bone Miner Res 30(3):499–509
Cejka D, Herberth J, Branscum AJ, Fardo DW, Monier-Faugere MC, Diarra D et al (2011) Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin J Am Soc Nephrol 6(4):877–882
Onyia JE, Helvering LM, Gelbert L, Wei T, Huang S, Chen P et al (2005) Molecular profile of catabolic versus anabolic treatment regimens of parathyroid hormone (PTH) in rat bone: an analysis by DNA microarray. J Cell Biochem 95(2):403–418
Guo J, Liu M, Yang D, Bouxsein ML, Saito H, Galvin RJ et al (2010) Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab 11(2):161–171
Yao GQ, Wu JJ, Troiano N, Insogna K (2011) Targeted overexpression of Dkk1 in osteoblasts reduces bone mass but does not impair the anabolic response to intermittent PTH treatment in mice. J Bone Miner Metab 29(2):141–148
Carrillo-Lopez N, Panizo S, Alonso-Montes C, Roman-Garcia P, Rodriguez I, Martinez-Salgado C et al (2016) Direct inhibition of osteoblastic Wnt pathway by fibroblast growth factor 23 contributes to bone loss in chronic kidney disease. Kidney Int 90(1):77–89
Anastasilakis AD, Polyzos SA, Avramidis A, Toulis KA, Papatheodorou A, Terpos E (2010) The effect of teriparatide on serum Dickkopf-1 levels in postmenopausal women with established osteoporosis. Clin Endocrinol (Oxf) 72(6):752–757
Viapiana O, Fracassi E, Troplini S, Idolazzi L, Rossini M, Adami S et al (2013) Sclerostin and DKK1 in primary hyperparathyroidism. Calcif Tissue Int 92(4):324–329
Wang Y, Sun Z (2009) Current understanding of klotho. Ageing Res Rev 8(1):43–51
Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J et al (2007) Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317(5839):803–806
Muñoz-Castañeda JR, Rodelo-Haad C, Pendon-Ruiz de Mier MV, Martin-Malo A, Santamaria R, Rodriguez M (2020) Klotho/FGF23 and Wnt signaling as important players in the comorbidities associated with chronic kidney disease. Toxins (Basel). 12(3):185
He W, Kang YS, Dai C, Liu Y (2011) Blockade of Wnt/beta-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol 22(1):90–103
Egan JB, Thompson PA, Vitanov MV, Bartik L, Jacobs ET, Haussler MR et al (2010) Vitamin D receptor ligands, adenomatous polyposis coli, and the vitamin D receptor FokI polymorphism collectively modulate beta-catenin activity in colon cancer cells. Mol Carcinog 49(4):337–352
He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y (2009) Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20(4):765–776
Hu MC, Kuro-o M, Moe OW (2013) Renal and extrarenal actions of Klotho. Semin Nephrol 33(2):118–129
Chen T, Mao H, Chen C, Wu L, Wang N, Zhao X et al (2015) The role and mechanism of α-Klotho in the calcification of rat aortic vascular smooth muscle cells. Biomed Res Int 2015:194362
Schuijers J, Clevers H (2012) Adult mammalian stem cells: the role of Wnt, Lgr5 and R-spondins. EMBO J 31(12):2685–2696
Zhu C, Zheng XF, Yang YH, Li B, Wang YR, Jiang SD et al (2016) LGR4 acts as a key receptor for R-spondin 2 to promote osteogenesis through Wnt signaling pathway. Cell Signal 28(8):989–1000
Kim KA, Zhao J, Andarmani S, Kakitani M, Oshima T, Binnerts ME et al (2006) R-Spondin proteins: a novel link to beta-catenin activation. Cell Cycle 5(1):23–26
Nam JS, Turcotte TJ, Smith PF, Choi S, Yoon JK (2006) Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J Biol Chem 281(19):13247–13257
Roman-Garcia P, Carrillo-Lopez N, Fernandez-Martin JL, Naves-Diaz M, Ruiz-Torres MP, Cannata-Andia JB (2010) High phosphorus diet induces vascular calcification, a related decrease in bone mass and changes in the aortic gene expression. Bone 46(1):121–128
Liao R, Wang L, Li J, Sun S, Xiong Y, Li Y et al (2019) Vascular calcification is associated with Wnt-signaling pathway and blood pressure variability in chronic kidney disease rats. Nephrology (Carlton). https://doi.org/10.1111/nep.13677
Rashdan NA, Sim AM, Cui L, Phadwal K, Roberts FL, Carter R et al (2019) Osteocalcin regulates arterial calcification via altered Wnt signaling and glucose metabolism. J Bone Miner Res. https://doi.org/10.1002/jbmr.3888
Leopold JA (2015) Vascular calcification: mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc Med 25(4):267–274
Zhu D, Mackenzie NC, Millán JL, Farquharson C, MacRae VE (2011) The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS ONE 6(5):e19595
Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K et al (2000) Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 87(7):E10–E17
Cannata-Andia JB, Roman-Garcia P, Hruska K (2011) The connections between vascular calcification and bone health. Nephrol Dial Transpl 26(11):3429–3436
Cannata Andia J, Carrillo-Lopez N, Rodriguez-Garcia M, Torregrosa JV (2014) Mineral and bone disorders in chronic kidney disease. In: Arici M (ed) Management of chronic kidney disease. Springer, Berlin, pp 223–239
Schlieper G, Schurgers L, Brandenburg V, Reutelingsperger C, Floege J (2016) Vascular calcification in chronic kidney disease: an update. Nephrol Dial Transpl 31(1):31–39
Cannata-Andia J, Martin-Carro B, Martin-Virgala J, Rodriguez-Carrio J, Bande-Fernandez J, Alonso-Montes C et al (2020) Chronic kidney disease—mineral and bone disorders: pathogenesis and management. Calcif Tissue Int. https://doi.org/10.1007/s00223-020-00777-1
Sabbagh Y, Graciolli FG, O’Brien S, Tang W, dos Reis LM, Ryan S et al (2012) Repression of osteocyte Wnt/beta-catenin signaling is an early event in the progression of renal osteodystrophy. J Bone Miner Res 27(8):1757–1772
Massy Z, Drueke T (2017) Adynamic bone disease is a predominant bone pattern in early stages of chronic kidney disease. J Nephrol 30(5):629–634
Behets GJ, Viaene L, Meijers B, Blocki F, Brandenburg VM, Verhulst A et al (2017) Circulating levels of sclerostin but not DKK1 associate with laboratory parameters of CKD-MBD. PLoS ONE 12(5):e0176411
Hansen S, Shanbhogue VV, Jørgensen NR, Beck-Nielsen SS (2019) Elevated bone remodeling markers of CTX and P1NP in addition to sclerostin in patients with X-linked hypophosphatemia: a cross-sectional controlled study. Calcif Tissue Int 104(6):591–598
Ardawi MS, Al-Kadi HA, Rouzi AA, Qari MH (2011) Determinants of serum sclerostin in healthy pre- and postmenopausal women. J Bone Miner Res 26(12):2812–2822
Roforth MM, Fujita K, McGregor UI, Kirmani S, McCready LK, Peterson JM et al (2014) Effects of age on bone mRNA levels of sclerostin and other genes relevant to bone metabolism in humans. Bone 59:1–6
Liu S, Song W, Boulanger JH, Tang W, Sabbagh Y, Kelley B et al (2014) Role of TGF-beta in a mouse model of high turnover renal osteodystrophy. J Bone Miner Res 29(5):1141–1157
Brandenburg VM, Verhulst A, Babler A, D’Haese PC, Evenepoel P, Kaesler N (2019) Sclerostin in chronic kidney disease-mineral bone disorder think first before you block it! Nephrol Dial Transpl 34(3):408–414
Shalhoub V, Shatzen E, Henley C, Boedigheimer M, McNinch J, Manoukian R et al (2006) Calcification inhibitors and Wnt signaling proteins are implicated in bovine artery smooth muscle cell calcification in the presence of phosphate and vitamin D sterols. Calcif Tissue Int 79(6):431–442
Woldt E, Terrand J, Mlih M, Matz RL, Bruban V, Coudane F et al (2012) The nuclear hormone receptor PPARgamma counteracts vascular calcification by inhibiting Wnt5a signalling in vascular smooth muscle cells. Nat Commun 3:1077
Deng D, Diao Z, Han X, Liu W (2016) Secreted frizzled-related protein 5 attenuates high phosphate-induced calcification in vascular smooth muscle cells by inhibiting the Wnt/ss-catenin pathway. Calcif Tissue Int. https://doi.org/10.1007/s00223-016-0117-7
Fang Y, Ginsberg C, Seifert M, Agapova O, Sugatani T, Register TC et al (2014) CKD-induced wingless/integration1 inhibitors and phosphorus cause the CKD-mineral and bone disorder. J Am Soc Nephrol 25(8):1760–1773
Ryan ZC, Ketha H, McNulty MS, McGee-Lawrence M, Craig TA, Grande JP et al (2013) Sclerostin alters serum vitamin D metabolite and fibroblast growth factor 23 concentrations and the urinary excretion of calcium. Proc Natl Acad Sci USA 110(15):6199–6204
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A et al (2014) Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 370(5):412–420
Evenepoel P, Cunningham J, Ferrari S, Haarhaus M, Kassim Javaid M, Lafage-Proust MH et al (2019) European consensus statement on the diagnosis and management of osteoporosis in chronic kidney disease stages 4 to 5D. Nephrol Dial Transpl. https://doi.org/10.1093/ndt/gfaa192
Evenepoel P, D’Haese P, Brandenburg V (2015) Sclerostin and DKK1: new players in renal bone and vascular disease. Kidney Int 88(2):235–240
De Maré A, Maudsley S, Azmi A, Hendrickx JO, Opdebeeck B, Neven E et al (2019) Sclerostin as regulatory molecule in vascular media calcification and the bone-vascular axis. Toxins (Basel) 11(7):428
McMahon LP, Roger SD, Levin A (2004) Development, prevention, and potential reversal of left ventricular hypertrophy in chronic kidney disease. J Am Soc Nephrol 15(6):1640–1647
Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE et al (2012) Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol 23(10):1725–1734
Cohn JN, Ferrari R, Sharpe N (2000) Cardiac remodeling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an international forum on cardiac remodeling. J Am Coll Cardiol 35(3):569–582
Fujii H (2018) Association between parathyroid hormone and cardiovascular disease. Ther Apher Dial 22(3):236–241
Acena A, Pello AM, Carda R, Lorenzo O, Gonzalez-Casaus ML, Blanco-Colio LM et al (2016) Parathormone levels are independently associated with the presence of left ventricular hypertrophy in patients with coronary artery disease. J Nutr Health Aging 20(6):659–664
Saleh FN, Schirmer H, Sundsfjord J, Jorde R (2003) Parathyroid hormone and left ventricular hypertrophy. Eur Heart J 24(22):2054–2060
Da Silva F, Massa F, Motamedi FJ, Vidal V, Rocha AS, Gregoire EP et al (2018) Myocardial-specific R-spondin3 drives proliferation of the coronary stems primarily through the Leucine Rich Repeat G Protein coupled receptor LGR4. Dev Biol 441(1):42–51
Zhao Y, Wang C, Hong X, Miao J, Liao Y, Zhou L et al (2018) An essential role for Wnt/beta-catenin signaling in mediating hypertensive heart disease. Sci Rep 8(1):8996
Zampetti S, Lucantoni F, Pacifico L, Campagna G, Versacci P, Pierimarchi P et al (2019) Association of OPG-RANKL ratio with left ventricular hypertrophy and geometric remodeling in male overweight/obese youths. J Endocrinol Invest 42(4):427–434
Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T et al (2011) FGF23 induces left ventricular hypertrophy. J Clin Invest 121(11):4393–4408
Bergmann MW (2010) WNT signaling in adult cardiac hypertrophy and remodeling: lessons learned from cardiac development. Circ Res 107(10):1198–1208
Acknowledgements
The authors wish to thank Instituto de Salud Carlos III (ISCIII; PI17/00715, PI19/00532, PI20/00753), the ISCIII Retic REDinREN (RD06/0016/1013, RD12/0021/0023, RD16/0009/0017 and RD16/0009/0018), Fondo Europeo de Desarrollo Regional (FEDER), Plan Estatal de I + D + I 2013–2016, Plan de Ciencia, Tecnología e Innovación 2013–2017 y 2018–2022 del Principado de Asturias (GRUPIN14-028, IDI-2018–000152), Fundación Renal Iñigo Álvarez de Toledo (FRIAT), and University of Oviedo. N.C.L. has been supported by FINBA-GRUPIN14-028 and IDI-2018-000152, L.M.A. by FINBA-ISCIII (PI17/00384), S.F.V. was supported by FINBA-ISCIII (PI17/00715) and S.P. by FINBA-IDI-2018-000152.
Author information
Authors and Affiliations
Consortia
Contributions
NCL and SP had the idea for the article, LMA and SFV performed the literature search and data analysis, SP, MND and JC drafted the article and NCL, MPRT, AD, JCA, MND and SP critically revised the article.
Corresponding authors
Ethics declarations
Conflict of interest
Natalia Carrillo-López, Laura Martínez-Arias, Sara Fernández-Villabrille, María Piedad Ruiz-Torres, Adriana Dusso, Jorge B. Cannata-Andía, Manuel Naves-Díaz, and Sara Panizo have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The EUROD Workgroup is an initiative of the chronic kidney disease-mineral bone disorder working group of the European Renal Association-European Dialysis and Transplant Association and International Osteoporosis Foundation.
Rights and permissions
About this article
Cite this article
Carrillo-López, N., Martínez-Arias, L., Fernández-Villabrille, S. et al. Role of the RANK/RANKL/OPG and Wnt/β-Catenin Systems in CKD Bone and Cardiovascular Disorders. Calcif Tissue Int 108, 439–451 (2021). https://doi.org/10.1007/s00223-020-00803-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-020-00803-2