Abstract
Purpose
The indications for adrenalectomy and feasibility of laparoscopic adrenalectomy for adrenal metastasis are controversial. This study aimed to compare the surgical outcomes between open adrenalectomy (OA) and laparoscopic adrenalectomy (LA) and to evaluate the prognostic factors for oncological outcomes of adrenal metastasis.
Materials and Methods
We conducted a retrospective chart review of 141 consecutive patients who underwent adrenalectomy for adrenal metastasis at Seoul National University Hospital from April 2005 to February 2021. Surgical and oncological outcomes were compared between OA and LA.
Results
OA was performed in 95 (67.4%) patients, and 46 (32.6%) patients underwent LA. Among the patients who underwent adrenalectomy without adjacent organ resection for adrenal tumors less than 8 cm, LA was associated with a shorter operation time (100.1 ± 48.8 vs. 158.6 ± 81.2, P = 0.001), less blood loss (94.8 ± 93.8 vs. 566.8 ± 1156.0, P = 0.034), and a shorter hospital stay (3.7 ± 1.3 vs. 6.9 ± 5.8, P = 0.003). For locoregional recurrence-free survival (LRRFS), on multivariate analysis, a positive pathological margin (hazard ratio [HR]: 5.777, P = 0.002), disease activity at the primary site (HR: 6.497, P = 0.005), other metastases (HR: 4.154, P = 0.015), and a relatively larger tumor size (HR: 1.198, P = 0.018) were significantly associated with poor LRRFS. Multivariate analysis indicated that metachronous metastasis (HR: 0.51, P = 0.032) was associated with a longer overall survival (OS), whereas a positive pathological margin (HR: 2.40, P = 0.017), metastases to other organs (HR: 2.08, P = 0.025), and a relatively larger tumor size (HR: 1.11, P = 0.046) were associated with a shorter OS.
Conclusions
LA is a feasible treatment option for adrenal metastasis in selected patients. The pathological margin, metastases to other organs, and tumor size should be considered in adrenalectomy for adrenal metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The adrenal gland is a common site for metastasis of malignancies from the lung, kidney, liver, breast, colon, and other primary sites [1]. The prevalence of adrenal metastases has been reported to be approximately 3.1% to 33% [2]. Patients with metastatic cancer to other organs are mostly considered to have stage IV disease, which needs palliative or supportive treatment. However, with improvements in systemic therapy, including chemotherapy and immunotherapy, several studies have demonstrated increased survival benefits of adrenalectomy compared with nonsurgical treatment alone for metastatic adrenal disease [3].
Depending on the origin of the primary cancer, heterogeneous long-term survival outcomes after adrenalectomy for adrenal metastasis have been reported. Krumeich et al. reported that lung cancer origin showed a durable survival outcome in median disease-free survival (DFS) (40 months; 1 year 64.8%, 5 year 42.9%) and overall survival (OS) (47 months; 1 year 80.2%, 5 year 35.2%) after adrenalectomy for metastatic adrenal disease [4]. Vlk et al. reported that for patients who underwent adrenalectomy for metastatic adrenal disease, renal and colon cancer patients had longer median survival times (37 months (IQR 13–82) and 35 months (IQR 12–83), respectively) than lung cancer patients (16 months (IQR 8–48)) [5].
According to current guidelines, adrenalectomy for metastatic adrenal disease can prolong DFS and OS in selected patient groups [1, 6]. Several factors, including the status of primary cancer, the number of metastatic sites, and the time interval between primary cancer to adrenal metastasis, need to be considered when selecting patients for this treatment [7]. Nevertheless, there are no definitive surgical indications for adrenalectomy for metastasis or prognostic factors related to survival outcomes.
With the development and advancement of minimally invasive surgery, the laparoscopic approach for adrenalectomy has been performed as the treatment of choice for metastatic disease [8]. However, if the adrenal tumor has malignant features, such as a large size, and is suspected to invade adjacent organs, conventional open surgery should be considered [9]. There are concerns about incomplete resection or tumor rupture, which may occur during the laparoscopic approach and could lead to the locoregional recurrence of metastatic cancer. Therefore, the indications for laparoscopic surgery for adrenal metastasis are unclear.
This study aimed to evaluate the prognostic factors of locoregional recurrence-free survival (LRRFS) and OS after adrenalectomy for adrenal metastasis and to compare the surgical outcomes between laparoscopic and conventional open adrenalectomy for adrenal metastasis.
Materials and methods
Patients
From April 2005 to February 2021, a retrospective chart review was performed of patients who underwent adrenalectomy for metastatic adrenal disease at Seoul National University Hospital. A total of 141 consecutive patients were identified. Patient clinicopathological data, operative approach, complications, locoregional recurrence, and survival data were collected. This study was approved by the Institutional Review Board of Seoul National University Hospital (IRB number: 2204-077-1316).
Definitions
The timing of adrenal metastasis from the primary tumor was defined as the period from the diagnosis date of primary cancer to the diagnosis date of adrenal metastasis; synchronous metastasis was defined as the detection of adrenal metastasis within 6 months after diagnosis of the primary tumor, and metachronous metastasis was defined as the detection of adrenal metastasis 6 months after diagnosis of the primary tumor. Locoregional recurrence was defined as radiological confirmation of recurrence in the previous adrenalectomy site. LRRFS was measured from the date of adrenalectomy to the date of the diagnosis of locoregional recurrence or the last follow-up. OS was defined as the interval from the date of adrenalectomy to the date of the last follow-up or death. Adjuvant systemic or radiation therapy was defined as any systemic or radiation treatment undergone after adrenalectomy.
Surgical intervention
Open, laparoscopic (or retroperitoneoscopic), and robotic adrenalectomies were performed by transperitoneal or retroperitoneoscopic approaches. To compare the conventional open approach and laparoscopic approach, patients were grouped based on the type of surgical approach; open and conversion to open surgeries were defined as the open approach group, and laparoscopic, retroperitoneoscopic and robotic surgeries were defined as the laparoscopic approach group. Surgeons chose the operative approach based on each patient’s clinical characteristics. Conventional open adrenalectomy was performed in patients with a relatively large tumor size, suspected invasion to adjacent organs or previous abdominal operation histories.
Statistical analysis
Continuous variables are presented as the mean with standard deviation or the median with interquartile range (IQR) as appropriate, and these data were analyzed by Mann–Whitney U tests. Categorical variables were analyzed using a chi-square test to compare the clinicopathological variables. Survival rates were calculated using Kaplan‒Meier survival analysis, with differences analyzed with the log-rank test. Univariate and multivariate Cox regression analyses were used to evaluate clinical characteristics associated with LRRFS and OS. P < 0.05 was defined as statistically significant. Statistical analyses were performed using R statistical software version 4.2.2 (http://www.R-project.org).
Results
Patient characteristics
During the study period, 141 patients underwent adrenalectomy for metastatic adrenal disease (Table 1). The most common type of cancer site was the kidney (39, 27.7%), followed by the liver (33, 23.4%), lung (24, 17.0%), colon (16, 11.3%), and others (29, 20.6%). The other primary cancers were sarcoma, breast cancer, stomach cancer, melanoma, etc. (Table S1). In patients with bilateral adrenal metastasis, synchronous contralateral adrenal metastasis was detected in 18 (51.4%) after the initial diagnosis of adrenal metastasis. Locoregional recurrence was detected in 38 (27.0%) patients, and the median locoregional recurrence-free interval was 18.0 (IQR 8.0–50.0) months. Unfortunately, 98 (69.5%) patients died during the study period. The median survival time after adrenalectomy was 25.0 (IQR 13.0–54.0) months.
Treatment of primary cancer and adrenal metastasis
The details of treatment for primary cancers and adrenal metastasis are shown in Table S2. Among 141 patients, open adrenalectomy was performed in 88 (62.4%) patients, and seven (4.7%) patients had a conversion to open adrenalectomy. Laparoscopic (or retroperitoneoscopic) adrenalectomy was performed in 42 (29.8%) patients; 36 patients underwent a laparoscopic approach, and 6 patients underwent a retroperitoneoscopic approach. Four (2.8%) patients underwent robotic adrenalectomy.
Comparison of surgical outcomes between open and laparoscopic adrenalectomy
A comparison of operative details between open and laparoscopic approaches is shown in Table 2. In terms of the surgical extent of adrenalectomy, a significant difference was found between the two groups (P < 0.001), namely, more patients in the open approach group had multiple organ resection. Compared to the laparoscopic approach, adrenalectomy with the open approach was associated with a significantly longer operation time (212.6 ± 113.5 min vs. 112.0 ± 63.5 min, P = 0.001), greater estimated blood loss (869.6 ± 1494.4 ml vs. 118.3 ± 123.0 ml, P = 0.001), longer postoperative hospital stay (8.9 ± 6.4 days vs. 4.3 ± 2.8 days, P = 0.001), and higher rates of postoperative complication rates (52.6% vs. 19.6%, P = 0.001). Locoregional recurrence was not different between the open and laparoscopic approaches (27 (29.0%) vs. 11 (23.9%), P = 0.524). Death was not different between the open and laparoscopic approaches (70 (73.7%) vs. 28 (60.9%), P = 0.121).
Comparison of surgical outcomes between open and laparoscopic adrenalectomy without adjacent organ resection for adrenal tumors smaller than 8 cm
To determine the surgical and oncological outcomes of laparoscopic adrenalectomy compared to the open approach, we performed subgroup analysis among the patients who did not undergo combined resection with other organs for adrenal tumors less than 8 cm (n = 73) (Table 3). Compared to open adrenalectomy (n = 34), laparoscopic adrenalectomy (n = 39) was associated with a significantly shorter operation time (158.6 ± 81.2 min vs. 100.1 ± 48.8 min, P = 0.001), less estimated blood loss (3.5 ± 1.7 cm vs. 2.8 ± 1.3 cm, P = 0.034), and a shorter postoperative hospital stay (6.9 ± 5.8 days vs. 3.7 ± 1.3 days, P = 0.003). There was no significant difference in the pathological margin, tumor size, overall postoperative complications, locoregional recurrence, or death between the groups.
Oncological outcomes: locoregional recurrence
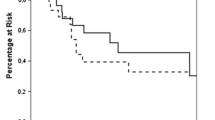
After adrenalectomy for adrenal metastasis, the 1-year LRRFS was 80.5%, and the 5-year LRRFS was 69.3% (Fig. S1). A multivariate Cox regression analysis indicated that a positive pathological margin (HR: 5.777, P = 0.002), the presence of disease activity at the time of adrenalectomy at the primary site (HR: 6.497, P = 0.005), other metastases (HR: 4.154, P = 0.015), and a relatively large tumor size (HR: 1.198, P = 0.018) were significantly associated with poor LRRFS (Table 4). The LRRFS of patients who underwent open adrenalectomy was not significantly different from that of patients who underwent laparoscopic adrenalectomy (P = 0.343) or adrenalectomy without adjacent organ resection for tumors smaller than 8 cm (P = 0.521) (Fig. 1).
Oncological outcomes: overall survival
After adrenalectomy for adrenal metastasis, the 1-year OS was 77.2%, and the 5-year OS was 31.4% (Fig. S1). On multivariate analysis, a longer OS was associated with metachronous adrenal metastasis from primary cancer (HR: 0.507, P = 0.032). A positive pathological margin (HR: 2.397, P = 0.017), presence of metastases to other organs at the time of adrenalectomy (HR: 2.078, P = 0.025), and a relatively large adrenal tumor (HR: 1.11, P = 0.046) were associated with a shorter OS (Table 5). The OS of patients who underwent open adrenalectomy was not significantly different from that of patients who underwent laparoscopic adrenalectomy (P = 0.278) or adrenalectomy without adjacent organ resection was performed for tumors smaller than 8 cm (P = 0.841) (Fig. 2).
Discussion
Adrenalectomy has been increasingly performed to treat adrenal metastases because resection of adrenal metastases may improve survival benefits [2, 10]. The current international guidelines of the American Association of Clinical Endocrinologists (AACE), American Association of Endocrine Surgeons (AAES) and European Society of Endocrine Surgeons (ESES) for the treatment of adrenal metastases suggest that adrenalectomy for adrenal metastasis is rarely indicated but may improve survival outcomes in a highly selected group of patients [6, 9]. Considerations should be given to pathological results, presentation timing from primary cancers, disease-free interval, and tumor size before adrenalectomy [1, 6]. The clinical outcome benefits can be explained by the recent advancement of minimally invasive surgery, improvement of systemic adjuvant therapy, and appropriate patient selection [8].
In the present study of the clinical outcomes of patients who underwent adrenalectomy for adrenal metastases, we found that laparoscopic adrenalectomy achieved favorable surgical outcomes and no significant difference in locoregional recurrence and death compared to open adrenalectomy. Since more patients in the open adrenalectomy group had multiple organ resection at the time of adrenalectomy, advanced disease requiring adjacent organ resection or reconstruction factors could inform the choice of the open approach rather than the laparoscopic approach. To avoid selection bias, we analyzed patients who underwent adrenalectomy without adjacent organ resection for adrenal tumors smaller than 8 cm and showed consistent clinical outcomes except for the postoperative complication rates.
Previous studies have reported the survival outcome of adrenalectomy for adrenal metastasis based on laparoscopic and open surgical approaches. Moreno et al. found that a patient group who underwent laparoscopic adrenalectomy for adrenal metastasis had a better median (IQR) survival compared to those who underwent open adrenalectomy (45.0 months; IQR 22.6–67.4 vs. 24.0 months; IQR 21.4–26.6, P = 0.008), which may be affected by several factors, including smaller tumor sizes, fewer patients who underwent extended adrenalectomy, and higher rates of R0 resection [11]. Goto et al. demonstrated that extra-adrenal metastasis at the time of adrenalectomy (HR 4.13, P = 0.022) and a positive surgical margin (HR 2.95, P = 0.049) were significantly associated with poor OS after laparoscopic adrenalectomy for adrenal metastasis [12]. Moreno et al. reported that synchronous metastasis was associated with a worse overall survival (HR 1.44, P = 0.041) [13]. However, other studies demonstrated no significant difference between synchronous and metachronous metastasis in survival outcomes [14, 15]. In the present study, we found that metachronous metastasis was associated with a better OS (HR 0.51, P = 0.032). Patients with metachronous metastasis may have indolent disease since the time to detection of adrenal metastasis from primary cancer in these patients is relatively long.
Although heterogeneous primary cancers may contribute as a bias to the survival outcome in our study cohort, durable survival was also observed after adrenalectomy in patients with adrenal metastasis, which seems to be consistent with previous studies [5, 16]. Based on the present study's findings, several prognostic factors for LRRFS and OS were identified. Positive resection margins, the presence of disease activity at the time of adrenalectomy, and a relatively large tumor size were significantly associated with lower rates of LRRFS and OS. Synchronous metastasis from primary cancer to the adrenal gland was an additional risk factor for poor OS. These findings may provide crucial evidence for deciding the surgical treatment plan for patients with adrenal metastasis of various primary cancers.
To the best of our knowledge, the present study is the first to analyze the clinical outcomes of patients who underwent adrenalectomy alone without adjacent organ resection for adrenal tumors smaller than 8 cm to minimize the selection bias of surgical approaches. Metastatic adrenal disease is often associated with a large tumor size or invasion of adjacent organs, which requires en bloc multivisceral resection via an open approach for a safe pathological margin around structures. This group of patients who underwent open adrenalectomy may have high complication rates and low oncological results. The size limit for the laparoscopic approach for large adrenal tumors has not been established, but recent data have demonstrated that if an adrenal tumor is larger than 8 cm, open adrenalectomy should be performed rather than a laparoscopic approach since it is related to worse surgical outcomes and risk of local invasion to surrounding organs [17,18,19,20]. Therefore, we compared the two groups of patients who underwent adrenalectomy alone with adrenal tumors smaller than 8 cm. We analyzed locoregional recurrence between the open and laparoscopic groups of patients. In our study, there were no significant differences in postoperative complications, pathological margins, or locoregional recurrence between the open and laparoscopic approaches.
This study has several limitations. The retrospective design and small number of patients from a single institution influence the results due to selection bias. Various types of primary cancer, which have different disease natures, can introduce heterogeneity in clinical outcomes. Over the long follow-up period, protocols for the management of metastatic adrenal disease were not consistent. The surgical approach regarding open or laparoscopic adrenalectomy was determined by the surgeons based on the patient’s clinical characteristics; tumor size and invasion to adjacent organs factored into the choice of operative approach. However, the present study is sufficient to analyze the surgical outcomes between open and laparoscopic adrenalectomy, including surgical margin, operation time, length of hospital stay, and postoperative complication. A future study of a large number of patients representing a multicenter prospective cohort is important to provide strong evidence.
In conclusion, laparoscopic adrenalectomy is a feasible treatment option for adrenal metastasis in selected patients. When performing adrenalectomy for adrenal metastasis, the pathological margin, disease activity at the time of adrenalectomy, and tumor size should be considered. For the decision regarding adrenalectomy for adrenal metastasis, multidisciplinary and global evaluations of these patients are critical.
References
Yip L, Duh QY, Wachtel H, Jimenez C, Sturgeon C, Lee C, Velazquez-Fernandez D, Berber E, Hammer GD, Bancos I, Lee JA, Marko J, Morris-Wiseman LF, Hughes MS, Livhits MJ, Han MA, Smith PW, Wilhelm S, Asa SL, Fahey TJ 3rd, McKenzie TJ, Strong VE, Perrier ND (2022) American Association of Endocrine Surgeons Guidelines for adrenalectomy: executive summary. JAMA Surg 157:870–877
Lam KY, Lo CY (2002) Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol (Oxf) 56:95–101
Kim SH, Brennan MF, Russo P, Burt ME, Coit DG (1998) The role of surgery in the treatment of clinically isolated adrenal metastasis. Cancer 82:389–394
Krumeich LN, Roses RE, Kuo LE, Lindeman BM, Nehs MA, Tavakkoli A, Parangi S, Hodin RA, Fraker DL, James BC, Wang TS, Solorzano CC, Lubitz CC, Wachtel H (2022) Survival after adrenalectomy for metastatic lung cancer. Ann Surg Oncol 29:2571–2579
Vlk E, Ebbehoj A, Donskov F, Poulsen PL, Rashu BS, Bro L, Aagaard M, Rolighed L (2022) Outcome and prognosis after adrenal metastasectomy: nationwide study. BJS Open 6:zrac047
Zeiger MA, Thompson GB, Duh QY, Hamrahian AH, Angelos P, Elaraj D, Fishman E, Kharlip J, American Association of Clinical E, American Association of Endocrine S (2009) The American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract 15(Suppl 1):1–20
Spartalis E, Drikos I, Ioannidis A, Chrysikos D, Athanasiadis DI, Spartalis M, Avgerinos D (2019) Metastatic carcinomas of the adrenal glands: from diagnosis to treatment. Anticancer Res 39:2699–2710
Strong VE, D’Angelica M, Tang L, Prete F, Gonen M, Coit D, Touijer KA, Fong Y, Brennan MF (2007) Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann Surg Oncol 14:3392–3400
Fassnacht M, Dekkers OM, Else T, Baudin E, Berruti A, de Krijger R, Haak HR, Mihai R, Assie G, Terzolo M (2018) European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 179:G1–G46
Vazquez BJ, Richards ML, Lohse CM, Thompson GB, Farley DR, Grant CS, Huebner M, Moreno J (2012) Adrenalectomy improves outcomes of selected patients with metastatic carcinoma. World J Surg 36:1400–1405
Moreno P, de la Quintana Basarrate A, Musholt TJ, Paunovic I, Puccini M, Vidal O, Ortega J, Kraimps JL, European Study Group for Metastatic A (2020) Laparoscopy versus open adrenalectomy in patients with solid tumor metastases: results of a multicenter European study. Gland Surg 9:S159–S165
Goto T, Inoue T, Kobayashi T, Yamasaki T, Ishitoya S, Segawa T, Ito N, Shichiri Y, Okumura K, Okuno H, Kawakita M, Kanaoka T, Terada N, Mukai S, Sugi M, Kinoshita H, Kamoto T, Matsuda T, Ogawa O (2020) Feasibility of laparoscopic adrenalectomy for metastatic adrenal tumors in selected patients: a retrospective multicenter study of Japanese populations. Int J Clin Oncol 25:126–134
Moreno P, de Quintana Basarrate A, Musholt TJ, Paunovic I, Puccini M, Vidal O, Ortega J, Kraimps JL, Bollo Arocena E, Rodriguez JM, Gonzalez Lopez O, Del Pozo CD, Iacobone M, Veloso E, Del Pino JM, Garcia Sanz I, Scott-Coombes D, Villar-Del-Moral J, Rodriguez JI, Vazquez Echarri J, Gonzalez Sanchez C, Gutierrez Rodriguez MT, Escoresca I, Nuno Vazquez-Garza J, Tobalina Aguirrezabal E, Martin J, Candel Arenas MF, Lorenz K, Martos JM, Ramia JM (2013) Adrenalectomy for solid tumor metastases: results of a multicenter European study. Surgery 154:1215–1222 (discussion 1222-1213)
Drake FT, Beninato T, Xiong MX, Shah NV, Kluijfhout WP, Feeney T, Suh I, Gosnell JE, Shen WT, Duh QY (2019) Laparoscopic adrenalectomy for metastatic disease: retrospective cohort with long-term, comprehensive follow-up. Surgery 165:958–964
Russo AE, Untch BR, Kris MG, Chou JF, Capanu M, Coit DG, Chaft JE, D’Angelica MI, Brennan MF, Strong VE (2019) Adrenal metastasectomy in the presence and absence of extraadrenal metastatic disease. Ann Surg 270:373–377
Wachtel H, Roses RE, Kuo LE, Lindeman BM, Nehs MA, Tavakkoli A, Parangi S, Hodin RA, Fraker DL, James BC, Carr AA, Wang TS, Solorzano CC, Lubitz CC (2021) Adrenalectomy for secondary malignancy: patients, outcomes, and indications. Ann Surg 274:1073–1080
Castillo OA, Vitagliano G, Secin FP, Kerkebe M, Arellano L (2008) Laparoscopic adrenalectomy for adrenal masses: does size matter? Urology 71:1138–1141
Townsend CM (2021) Sabiston textbook of surgery E-Book: the biological basis of modern surgical practice. Elsevier Health Sciences, New York
Boylu U, Oommen M, Lee BR, Thomas R (2009) Laparoscopic adrenalectomy for large adrenal masses: pushing the envelope. J Endourol 23:971–975
Mihai R (2019) Open adrenalectomy. Gland Surg 8:S28–S35
Funding
Open Access funding enabled and organized by Seoul National University. This study was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare of the Republic of Korea (Project No. HI22C0049), and a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (Grant Numbers: 2021R1F1A1055710).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
JungHak Kwak, Hye Lim Bae, Younghoon Jung, Jaebong Choi, Hyeonuk Hwang, Jung Hee Kim, Su-jin Kim, and Kyu Eun Lee have no conflicts of interest or financial ties to disclose.
Informed consent
Informed consent was exempted by the Institutional Review Board of Seoul National University Hospital due to the retrospective nature of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kwak, J., Bae, H.L., Jung, Y. et al. Comparative outcomes and prognostic indicators in adrenalectomy for adrenal metastasis. Surg Endosc 38, 1884–1893 (2024). https://doi.org/10.1007/s00464-024-10691-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-024-10691-4