Abstract
Introduction
Gastrointestinal anastomoses with classical sutures and/or metal staples have resulted in significant bleeding and leak rates. This multi-site study evaluated the feasibility, safety, and preliminary effectiveness of a novel linear magnetic compression anastomosis device, the Magnet System (MS), to form a side-to-side duodeno-ileostomy (DI) diversion for weight loss and type 2 diabetes (T2D) resolution.
Methods
In patients with class II and III obesity (body mass index [BMI, kg/m2] ≥ 35.0– ≤ 50.0 with/without T2D [HbA1C > 6.5%]), two linear MS magnets were delivered endoscopically to the duodenum and ileum with laparoscopic assistance and aligned, initiating DI; sleeve gastrectomy (SG) was added. There were no bowel incisions or retained sutures/staples. Fused magnets were expelled naturally. Adverse events (AEs) were graded by Clavien-Dindo Classification (CDC).
Results
Between November 22, 2021 and July 18, 2022, 24 patients (83.3% female, mean ± SEM weight 121.9 ± 3.3 kg, BMI 44.4 ± 0.8) in three centers underwent magnetic DI. Magnets were expelled at a median 48.5 days. Respective mean BMI, total weight loss, and excess weight loss at 6 months (n = 24): 32.0 ± 0.8, 28.1 ± 1.0%, and 66.2 ± 3.4%; at 12 months (n = 5), 29.3 ± 1.5, 34.0 ± 1.4%, and 80.2 ± 6.6%. Group mean respective mean HbA1C and glucose levels dropped to 1.1 ± 0.4% and 24.8 ± 6.6 mg/dL (6 months); 2.0 ± 1.1% and 53.8 ± 6.3 mg/dL (12 months). There were 0 device-related AEs, 3 procedure-related serious AEs. No anastomotic bleeding, leakage, stricture, or mortality.
Conclusion
In a multi-center study, side-to-side Magnet System duodeno-ileostomy with SG in adults with class III obesity appeared feasible, safe, and effective for weight loss and T2D resolution in the short term.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Metabolic/bariatric surgery (MBS) is substantially more effective and durable in achieving weight loss and type 2 diabetes control than conventional lifestyle change and medication [1,2,3]. MBS is also highly cost effective in managing patients with T2D with/without obesity, saving insurers > $76.5 million in T2D medication costs in the first post-MBS year in one state alone [4]. MBS is safe, with approximately the same short-term morbidity of common procedures such as cholecystectomy and appendectomy [5, 6]. Since the introduction of laparoscopic technique in Roux-en-Y gastric bypass (RYGB) by Wittgrove and Clark (1993) [7, 8], minimally invasive surgery (MIS) has continued to reduce operative and long-term MBS risk [9, 10] and encompass novel modes of access (e.g., endoscopic, natural orifice transluminal endoscopic surgery [NOTES], robotic), technique simplification (e.g., single-anastomosis, staging/dividing complex operations), and innovative technology (e.g., intragastric balloon, gastric electrical stimulation, duodenojejunal bypass liner) [11, 12].
Forming and protecting the anastomosis must be an essential minimally invasive MBS focus. Compression anastomosis (CA) creation, performed as early as 1826 with a sutureless metal bowel ring by Denan [13], has been applied effectively in twenty-first century duodeno-ileal (DI) and duodeno-colic (DC) preclinical procedures by Gagner et al. [14, 15], and clinically, in the biofragmentable anastomotic ring (BAR) by Hardy et al., and the nickel-titanium ring and clip (NiTi CAR/CAC) reported by Nudelman et al. [16,17,18]. Although CA devices have shown efficacy equivalent to conventional sutures and staples [19, 20], they require fixation in the bowel with sutures, staples, clips, or glue which may stimulate an inflammatory response.
Alternatively, magnetic CA (MCA) eliminates sharp bowel division followed by sewing/stapling to form the anastomosis, relying instead on magnetic compression with no anatomical fixation. MCA is initiated intraoperatively by alignment of magnets across two GI bowel segments. The gradually fusing magnets necrose and slough the interposed tissue over several weeks while healing occurs at the magnets’ circumference. Magnets are expelled naturally leaving no foreign materials in the body [20, 21].
Few MCA outcomes in MBS have been reported. We performed a preclinical feasibility study of MCA [22] with favorable outcomes that supported conduct of a first-in-human (FIH) trial of the novel Magnet Anastomosis System (MS) in a side-to-side duodeno-ileal (DI) diversion with an added sleeve gastrectomy (SG) [23]. Magnetic DI diversion performed side-to-side coupled with SG may provide the strong weight loss and associated medical condition (AMC) resolution of duodenal switch (DS) without its hypoabsorption, and the added MIS advantage of a single-anastomosis DI with SG (SADI-S). In the current study, the scope of the FIH safety investigation was expanded to examine the feasibility, ongoing safety, and early efficacy of side-to-side MS DI + SG in a small cohort in multiple centers.
Patients and methods
Study design and protocol
A prospective observational open-label evaluation (Clinicaltrials.gov NCT#05,322,122) of the investigational Magnet Anastomosis System (MS; GT Metabolic Solutions, San Jose, CA) used in side-to-side magnetic DI for patients with class III obesity (body mass index [BMI, kg/m2] ≥ 40.0 to ≤ 50.0) was conducted in two stages. Stage 1 (n = 5) evaluated FIH feasibility and safety outcomes at a single site (Republic of Georgia); stage 2 (the current study) evaluated feasibility, ongoing safety, and efficacy (n = 24) in the first site plus two additional centers (Georgia, n = 5; Belgium, n = 9; Spain, n = 10) at interim time points through one year.
Ethics
The protocol incorporated guidelines for use of investigational devices. The Ethics Committees and IRBs of the three centers approved the protocol and monitored the progress of the study. Adverse events (AEs) and severe AEs (SAEs) were analyzed during the conduct of the study by an independent data and safety monitoring board (DSMB). The Helsinki Declaration and ISO14155 regulations and 21 CFR Good Clinical Practices ensured patients’ protection and wellbeing throughout the study.
Inclusion and exclusion
The surgeon and surgical team at the participating centers introduced potential patients to the study aims, procedure, and expected outcomes. Based on a reasonable understanding of the background, each participating patient provided written informed consent for study participation.
Inclusion criteria required patients to be 18–65 years old with a BMI of ≥ 30.0 to ≤ 50.0 kg/m2 and either: type 2 diabetes (T2D: HbA1C ≥ 6.5% on diabetes medication) with no prior MBS; or prior sleeve gastrectomy (SG) ≥ 12 months previously with T2D with/without weight regain; or have a BMI ≥ 40 kg/m2 and be considering undergoing laparoscopic SADI-S where DI was performed side to side rather than end to side. Patients agreed not to undergo additional MBS or reconstructive surgery for 1 year; females agreed not to become pregnant and to use contraception for 1 year. Prescription or over-the-counter weight-loss medication and non-steroidal anti-inflammatory drugs were prohibited for 14 days before the procedure and during the study.
Exclusions to participation included: type 1 diabetes; uncontrolled T2D, dyslipidemia, hypertension, and sleep apnea; injectable insulin use; prior non-MBS intestinal, colonic, or duodenal surgery; prior trauma, implant of prostheses, disease, scarring, abnormal anatomy, or genetic preconditions that prevented or contraindicated the study procedure; refractory gastroesophageal reflux disease (GERD), helicobacter pylori positive and/or active ulcer disease; large hiatal hernia; inflammatory bowel disease or colonic diverticulitis; an anomaly or condition precluding access by gastroscopy or laparoscopy; implantable pacemaker or defibrillator; untreated or poorly controlled psychiatric illness; substance abuse history; pregnancy, breastfeeding, or unwillingness to use a proven contraception method; an AMC that presented a safety concern; an interventional procedure 30 days before/after the procedure; stroke/TIA within 6 months of study consent; chronic anticoagulation therapy (except aspirin); active infection requiring antibiotics; inability to comply with the follow-up schedule; participation in a separate clinical investigation; known allergies to device components or contrast media; limited life expectancy due to terminal disease; positive Covid-19 test before the procedure; and any condition that might prevent 360-day follow-up.
MS DI procedure
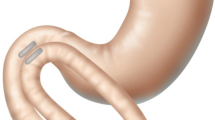
Comprised of a pair of linear BC42 neodymium magnets (0.75″ length × 0.25″ width × 0.125″ thickness) with 2.3-mm-offset perimeter flange and Ti-6Al-4 V ELI grade-23 titanium casing (KJ Magnetics, Pipersville, PA) (Fig. 1), the MS was designed specifically to effect side-to-side DI diversion. The full endoscopic technique with laparoscopic assistance for side-to-side MS DI has been described [23]. In brief, after placing a marker in the ileum 250 cm from the cecum, the first (distal) MS magnet is transported orogastrically to the ligament of Treitz, then directed through the jejunal lumen by a magnetized positioning device to the marked ileal position (Fig. 2a); non-magnetic bowel forceps raise the distal ileal magnet over the transverse colon, anterior and latero-lateral to the post-pyloric duodenum, where the proximal MS magnet has been delivered endoscopically and the two magnets are aligned (Fig. 2b); Petersen’s defect is closed and an SG is performed (Fig. 2c).
Side-to-side Magnet System (MS) duodeno-ileostomy: the distal MS magnet is endoscopically positioned at the ligament of Treitz and directed through the jejunal lumen to the ileum with laparoscopic assistance by a magnetic positioning device (a); the distal magnet is raised anterior and latero-lateral to the post-pyloric duodenum to align with the endoscopically delivered proximal MS magnet (b); Petersen’s defect is closed and a sleeve gastrectomy is performed (c). In 5–7 days magnets are fused; in several weeks, the patent DI anastomosis is formed; the magnet pair is expelled naturally, and food transits through duodenum and ileum (d)
In 5–7 days, magnets are fused. Within several weeks, a robust, patent anastomosis is formed and the magnet pair detaches from the DI site and is expelled naturally enabling the transit of food through the duodenal lumen and the ileal lumen (Fig. 2d).
Post-procedure care
Patients’ hemodynamic conditions and cardiac rhythm were monitored closely following the procedure. Correct MS placement in the abdomen was confirmed on postoperative day 1 by x-ray, and by fluoroscopy using barium or gastrographin. Before discharge, patients discussed the postoperative diet with the dietitian or nutritionist. Patients attended six follow-up visits (day 30, 60, 90, 180, 270, and 360), returning as needed for consultations.
Endpoints
Feasibility was the primary endpoint assessed by (1) correct technical positioning of the MS magnets, (2) magnet expulsion with no device-related AEs requiring surgical reintervention, and (3) patent DI anastomosis creation verified radiologically and fluoroscopically. If the MS performed appropriately in ≥ 80% of patients, the primary feasibility endpoint was considered achieved. Incidence of device- and procedure-related AEs/SAEs at 6 months and 1 year represented the primary safety endpoints. Adverse events were characterized using the Clavien-Dindo Classification (CDC) [24].
Secondary endpoints were measures of MBS efficacy including weight loss and improvement in T2D as measured by reduction or elimination of medications and improvement of metabolic markers at 6- and 12-month follow-up including the proportion of patients with > 5.0% total weight loss (TWL). TWL was calculated by: ([initial weight − follow-up weight]/[initial weight] × 100); excess weight loss (%EWL) by: ([initial weight − follow-up weight]/[initial weight – ideal body weight] × 100); and BMI loss: (initial BMI – post-intervention BMI).
Statistical analysis
Descriptive statistics were calculated using the SPSS statistical package (version 20.0; IBM, Chicago, IL). Primary endpoints were represented by categorical variables and reported using frequencies and percentages. Summary statistics for continuous variables were reported using means and standard error of the mean (SEM), ranges and/or 95% CIs. Group mean changes in weight and metabolic parameters were assessed using the paired samples t-test; alpha was set at p < 0.05.
Results
Patient characteristics
Between November 22, 2021 and July 18, 2022, 24 patients (83.3% female; 75.0% Caucasian) with a mean age of 43.8 ± 1.8 years (range 28–59) underwent side-to-side MS DI + SG. The group mean baseline absolute weight was 121.9 ± 3.3 kg (97–155) with a BMI of 44.4 ± 0.8 (36.8–50.9) (Table 1). Nine patients (37.5%) were diagnosed with T2D, most on medications, at study enrollment. (Three patients, one in an included site, two with 3-month follow-up in a site that was not included in this study, did not receive an SG concurrent with the DI and are incorporated in an independent report.)
Feasibility
The MS successfully met feasibility criteria: placement of the device with alignment of magnets, creation of a patent anastomosis confirmed radiologically (Fig. 3), and passage of the paired magnets without AEs requiring re-intervention was achieved in all 24 (100.0%) patients. The time to device expulsion (subject to self-report) ranged from 14 to 92 days with a median of 48.5 days (n = 22; mean 48.2 ± 4.7 days). Two patients indicated they did not note the natural passage of the magnets; however, expulsion was subsequently confirmed through imaging.
Secondary efficacy endpoints
Weight
Group mean body weight (n = 24) was reduced from 121.9 ± 3.3 kg at baseline to 87.8 ± 2.8 kg at day 180, representing an overall mean weight change of 34.2 ± 1.6 kg (p < 0.001) (Table 2). For the five patients who reached the 360-day follow-up, mean body weight fell to 77.6 ± 4.7 kg, an overall reduction of 40.0 ± 3.1 kg (p < 0.001). Figure 4a and b depict individual patient weight-loss trajectories to day 180 (n = 24) and day 360 (n = 5), respectively. As presented in Table 2 and Fig. 5a, BMI fell significantly from 44.4 ± 0.8 at baseline to 32.0 ± 0.8 (p < 0.001) at day 180 (n = 24) and further to 29.3 ± 1.5 at day 360 (n = 5), for an overall BMI reduction of 15.1 ± 1.0 kg/m2 (p < 0.001). Figure 5b and c depict concomitant progressive increases in TWL and EWL. At day 180, mean TWL and EWL were 28.1 ± 1.0 and 66.2 ± 3.4, respectively (n = 24); at day 360, 34.0 ± 1.4 and 80.2 ± 6.6 (n = 5). All 24 patients (100.0%) surpassed the efficacy criterion of > 5.0% TWL at day 180, and achieved significantly more success with TWL ranging from 20. to 37.6%.
Metabolic indicators
Figure 6a and b depict changes in HbA1C (n = 19) and glucose levels at postoperative day 180 (n = 21) and day 360 (n = 4), respectively; blood draw compliance was not complete for every patient. Group mean HbA1C level was reduced from 6.2 ± 0.3% at baseline to 5.1 ± 0.2% at day 180, representing a significant overall mean change of 1.1 ± 0.4% (p < 0.05) (Table 2). For the 4 patients who reached the 360-day follow-up timepoint, HbA1C fell to 4.8 ± 0.2, an overall reduction of 2.0 ± 1.1%. Mean glucose level was reduced from 111.3 ± 6.1 mg/dL to 86.5 ± 3.5 mg/dL at day 180, a significant overall mean change of 24.8 ± 6.6 mg/dL (p < 0.001); at day 360 glucose fell to 87.3 ± 6.3 mg/dL, an overall reduction of 53.8 ± 6.3 mg/dL (Table 2).
Mean changes following side-to-side Magnet System duodeno-ileostomy with sleeve gastrectomy in (a) HbA1C (%): reduction at 6 months, 1.1 ± 0.4% (n = 19) and at 12 months, 2.0 ± 1.1% (n = 4); and in (b) blood glucose (mg/dL), reduction at 6 months, 24.8 ± 6.6% (n = 21) and at 12 months, 53.8 ± 6.3% (n = 4)
Seven of 9 patients diagnosed with T2D were under treatment with antidiabetics at enrollment. One hundred percent (7/7) of T2D patients noted less need for medication, with six patients (85.0%) ceasing all medications and one patient reducing medication at day 180. Specifically, four patients stopped medications at enrollment. One patient entered the study taking 3 diabetes medications but stopped 2 immediately (empagliflozin, gliclazide), and stopped the third (metformin) 2 weeks post procedure. Another patient on 2 medications immediately stopped semaglutide, continuing metformin until discontinuing it at 4 months post procedure. The last of the seven patients was on 3 medications and stopped 2 immediately (liraglutide and dapagliflozin), continuing metformin through follow-up. Of the two patients entering with no prescribed T2D medication, one remained off medication, and the other started metformin 10 days post procedure, continuing through follow-up.
Safety
There were no anastomotic bleeds, leaks, obstruction, infection and no deaths throughout the study. In 21 patients, a total of 57 adverse events was reported, none related to the device. AEs comprised 25 CDC grade I (43.8%), 22 grade II (38.6%), and 10 grade III (17.5%). Included among minor and moderate CDC grade I and II AEs were cases of Covid-19, nausea, diarrhea, vomiting, abdominal pain, an esophageal mucosal tear from blind insertion of the overtube, wound pain, vitamin/mineral deficiency, dehydration, pulmonary and urinary infection, and reflux.
Three SAEs (5.3%, 3/57) were determined to be related to the study procedure, none to the MS. One event was a urinary tract infection with mild fever on postoperative day one; the patient was treated with antibiotics and recovered without sequelae. The second event was a small jejunal obstruction due to an internal hernia in the mesentery, despite the per-protocol closure of the mesenteric defect during the procedure. Laparoscopic repair was performed, and the patient discharged the next day without sequelae. The third event was a pelvic fluid collection that continued for 2 months. After transvaginal drainage, the patient recovered and was in good condition. The 8 SAEs of CDC grade II or III are detailed in Table 3. There were no grade IV or V AEs.
Discussion
The side-to-side Magnet Anastomosis System DI procedure was a feasible technique for surgeons and multidisciplinary MBS teams at three sites. The linear MS device was readily placed, well tolerated in the bowel, and successful in forming patent, sizeable, enduring duodeno-ileal bipartitions without anastomotic leakage through 1-year follow-up. In addition, duodeno-ileostomies were incisionless and suture/staple free. The fused magnets were expelled naturally without migration, erosion, or pair separation. At 6- and 12-month follow-up, substantial weight loss and BMI reduction were observed with 86.0% T2D resolution/100.0% improvement (all T2D medications eliminated [n = 6]) or reduced [n = 1]). Over 1 year, there were 57 total AEs; although none were device related, 8 were determined to be SAEs of CDC grade II or III.
The clinical feasibility of using magnets to effect patent anastomoses in a side-to-side DI can only be directly compared at this time to a feasibility study in 8 patients (4/8 females, median BMI 38.8) of “self-forming magnets (SFM)” by Schlottmann et al. In their series with 1-year follow-up, two octagonally shaped magnets were aligned in the duodenum and ileum, and expelled naturally without device-related AEs. Patent anastomoses were achieved with the proximal SFM magnet delivered by upper endoscopy and the distal magnet by laparoscopy through a 5-mm ileotomy closed with absorbable suture [25]. The SFM enterotomy introduces potential for a leak at the DI site; whereas the MS is positioned without incision or sutures/staples. The devices cannot be compared in terms of efficacy as MS DI was combined with SG in the current study.
The end-to-side SADI-S is the closest comparator to the side-to-side MS DI + SG. Operative time, weight loss, and metabolic outcomes in the magnetic DI + SG study were effective and roughly equivalent to those of SADI-S in three recent observational studies and a meta-analysis. In a prospective short-term comparative study of SADI-S (n = 42) and DS (n = 20) by Andalib et al. (2021), operative time was briefer with primary SADI-S (median 211 min) than for DS (250 min), p = 0.05; whereas, in the current study of side-to-side MS DI + SG, operative time was less (median of 130 min) than for both SADI-S and DS in the Andalib report [26]. Although no randomized controlled trial (RCT) evidence for SADI-S exists currently, a systematic review and meta-analysis by Verhoeff et al. (2022) of 16 studies, 1704 (51.3%) of primary SADI-S compared with other hypoabsorptive procedures, the weighted mean operative time of 103.6 min [27] was less than that of the other hypoabsorptive operations compared in their study and that of the current MS DI + SG operation.
The median EWL of primary SADI-S in Andalib et al. at 6- and 12-month follow-up was, respectively, 72.6% and 86.8% with BMI of 32.9 and 29.4 [26]. In comparing SADI-S vs DS at 2-year follow-up, Verhoeff et al. found their TWL and BMI similar (respectively, 37.3% and 29.8 vs 37.6% and 31.1) [27]. In the current study of magnetic DI + SG, patients with 1-year follow-up (n = 5) had a somewhat lower TWL of 34.0% (EWL 80.2%), and virtually the same BMI of 29.3 found in the Verhoeff et al. meta-analysis. Sanchez‑Pernaute et al., innovators of the SADI-S, recently reported (2022) the largest consecutive series (n = 164) of SADI-S (5-year follow-up [n = 139, 84.7%] with some patients followed through 10 years), respective 1-year TWL, EWL, and BMI were 42.0%, 95.5%, and 26.5 [28]. Whereas, at 1 year, magnetic DI + SG TWL and EWL were markedly lower than those of Sanchez-Pernaute et al., they were still highly significant in our small cohort.
T2D resolution in the current study was 86.0% (n = 6/7 off all medications) at 6 months and 100.0% at 1 year (n = 3/3 off all medications). In the comparative study of SADI-S and DS by Andalib et al., the T2D resolution rate was a median of 91.0% (n = 21/23 at 10 months) and 50.0% after DS (n = 3/6 at 14 months) [26]. The Verhoeff et al. meta-analysis weighted mean remission rate for T2D of 87.4% following SADI-S (measured at longest follow-up, 28.8 months) [27] was similar to that of the current study at 1-year follow-up notwithstanding its small sample size. In a prospective multicenter study of primary SADI-S by Cottam et al. (2020) at 1-year follow-up, in SADI-S patients, T2D in 54/57 of patients (96.0%) was either resolved or improved with continued medications [29]. In relation to recent studies of SADI-S (and SADI-S vs DS), side-to-side MS DI + SG appeared to achieve a comparable rate of T2D resolution.
Side-to-side MS DI + SG had a 0.0% anastomotic leak rate through 1-year follow-up. In the meta-analysis by Verhoeff et al., 10 duodeno-ileal anastomosis leaks (6/16 studies) were reported [27]. Andalib et al. described 1 leak two days after SADI-S at the duodeno-ileal site requiring reoperative repair and drainage [24]; and in Sanchez-Pernaute et al., 2 leaks at the duodeno-ileostomy, one requiring reoperation [28]. While the current study had technical success in magnetic anastomosis formation without leak and reoperations, the SAEs sustained represent an area of challenge requiring future improvement.
Creating and protecting a patent, continuously functioning, non-inflammatory anastomosis is critical to MBS. Compromised anastomotic healing may cause a leak, resulting in potential sepsis or death [30, 31]. The human burden and financial costs are significantly elevated for MBS patients who suffer anastomotic leaks [32]. Use of sutures/staples for anastomosis formation may negatively impact the blood supply in GI tissues. If a stapler is too small for the site, anastomotic stenosis can result. An intestinal fold inadvertently embedded in a sutured/stapled anastomosis can form granulation tissue postoperatively. In addition, GI staples will affect subsequent computed tomography and magnetic resonance imaging examination and treatment [18].
MIS approaches that employ delayed anastomosis technology (DAT), a concept developed by Gagner (2021), aim to alleviate acute and chronic inflammatory responses associated with instant intraoperative anastomosis creation using foreign materials [33]. The MS is a device and approach that embodies DAT. The unhurried DAT paradigm is nonetheless efficient, as gradual healing optimizes conditions for collagen deposition. Patients may require less anesthesia, operative time, and hospital stay, and need fewer reinterventions due to anastomotic complications.
Technical difficulties associated with stapled/sutured anastomoses may be mitigated by magnets combined with single-anastomosis procedures. Feasibility and therapeutic value of employing MCA in other digestive tract indications are being explored and have shown early effectiveness, such as in complex pediatric ileal resection where temporary magnets have been used to create inter-intestinal anastomoses over 5–7 days to treat intestinal invagination. In addition to magnets’ mechanical induction of an anastomosis, evidence suggests that their magnetic field provides physiotherapeutic stimulation of the microcirculation contributing to anastomotic tissue regeneration. [34, 35]. In the context of complex esophageal atresia, endoscopic MCA has been used to achieve delayed primary esophageal repair [36]. Also, in small cohorts, GI fistula, cancer, obstruction, ulcer, and patients with complex abdominal histories have been treated feasibly and safely with MCA [37, 38].
Limitations
A limitation of this study was its lack of a comparison group. The cohort was multi-national but relatively small, and the design observational; therefore, generalization of efficacy results beyond establishing preliminary trends was not appropriate. The current study protocol was not designed to compare the change in nutritional variables experienced following side-to-side MS DI + SG; doing so in future research would elucidate the nutritional efficacy of this procedure compared with that of SADI-S.
Conclusions
MCA can offer a further advancement of minimally invasive MBS. In a small multi-center study, the incisionless, sutureless Magnet System was a feasible and safe method of anastomosis formation in side-to-side duodeno-ileal diversion. Side-to-side Magnet System DI + SG proved effective in reducing obesity and T2D at interim timepoints through 1-year follow-up. Larger cohorts and RCT designs are needed to assess MCA safety and effectiveness.
References
Eisenberg D, Shikora SA, Aarts E, Aminian A, Angrisani L, Cohen RV et al (2022) 2022 American society for metabolic and bariatric surgery (ASMBS) and international federation for the surgery of obesity and metabolic disorders (IFSO): indications for metabolic and bariatric surgery. Surg Obes Relat Dis 18(12):1345–1356. https://doi.org/10.1016/j.soard.2022.08.013
Kirwan JP, Courcoulas AP, Cummings DE, Goldfine AB, Kashyap SR, Simonson DC, Arterburn DE, Gourash WF, Vernon AH, Jakicic JM, Patti ME, Wolski K, Schauer PR (2022) Diabetes remission in the alliance of randomized trials of medicine versus metabolic surgery in type 2 diabetes (ARMMS-T2D). Diabetes Care 45(7):1574–1583. https://doi.org/10.2337/dc21-2441.PMID:35320365;PMCID:PMC9490448
Schauer P, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA et al (2017) Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med 376(7):641–651
Varban OA, Hassett KP, Yost M, Carlin AM, Ghaferi AA, Finks JF, Ehlers AP (2023) Financial impact of metabolic surgery on prescription diabetes medications in Michigan. JAMA Surg. https://doi.org/10.1001/jamasurg.2022.7749
Aminian A, Brethauer SA, Kirwan JP, Kashyap SR, Burguera B, Schauer PR (2015) How safe is metabolic/diabetes surgery? Diabetes Obes Metab 17(2):198–201. https://doi.org/10.1111/dom.12405
Chang S, Stoll CRT, Song J, Varela JE, Eagon CJ, Colditz GA (2014) The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA Surg 149(3):275–287. https://doi.org/10.1001/jamasurg.2013.3654
Wittgrove AC, Clark GW, Tremblay LJ (1994) Laparoscopic gastric bypass, Roux en-Y: preliminary report of five cases. Obes Surg 4:353–357
Wittgrove AC, Clark GW, Schubert KR (1996c) Laparoscopic gastric bypass, Roux-en-Y: technique and results in 75 patients with 3–30 months follow-up. Obes Surg 6(6):500–504. https://doi.org/10.1381/096089296765556412
Gagner M, Gentileschi P, de Csepel J, Kini S, Patterson E, Inabnet WB et al (2002) Laparoscopic reoperative bariatric surgery: experience from 27 consecutive patients. Obes Surg 12(2):254–260. https://doi.org/10.1381/096089202762552737
Sundbom M (2014) Laparoscopic revolution in bariatric surgery. World J Gastroenterol 20(41):15135–15143. https://doi.org/10.3748/wjg.v20.i41.15135.PMID:25386062;PMCID:PMC4223247
Bhandari M, Fobi MAL, Buchwald JN, The Bariatric Metabolic Surgery Standardization Working Group (2019) Standardization of bariatric metabolic procedures: world consensus meeting statement. Obes Surg. https://doi.org/10.1007/s11695-019-04032-x
Batchelder AJ, Williams R, Sutton C, Khanna A (2013) The evolution of minimally invasive bariatric surgery. J Surg Res 183(2):559–566. https://doi.org/10.1016/j.jss.2013.02.036
Amat C (1895) Appareils a sutures: Les viroles de denans; les points de Bonnier; Les boutons de Murphy. Arch Med Pharmacie Militaires Paris 25:273–285
Gagner M (2015) Safety and efficacy of a side-to-side duodeno-ileal anastomosis for weight loss and type-2 diabetes: duodenal bipartition, a novel metabolic surgery procedure. Ann Surg Innov Res 14(9):6
Gagner M (2017) Side-to-side duodeno-colic anastomosis provides dramatic weight loss. A potentially strong anti-diabetic operation for type-2 diabetes. Minerva Chir 72(3):169–177
Hardy TG Jr, Pace WG, Maney JW, Katz AR, Kaganov AL (1985) A biofragmentable ring for sutureless bowel anastomosis. An Exp Study Dis Colon Rectum 28:484–490
Nudelman IL, Fuko VV, Morgenstern S, Giler S, Lelcuk S (2000) Gastrointestinal anastomosis with the nickel-titanium double ring. World J Surg 24:874–877
Xu Z, Zhen Y (2021) Magnets for colorectal anastomosis. Chapter 6. In: Gagner M (ed) Magnetic Surgery. Springer, New York NY, 43-59
Slesser AA, Pellino G, Shariq O, Cocker D, Kontovounisios C, Rasheed S, Tekkis pp. (2016) Compression versus hand-sewn and stapled anastomosis in colorectal surgery: a systematic review and meta-analysis of randomized controlled trials. Tech Coloproctol 20(10):667–676. https://doi.org/10.1007/s10151-016-1521-8
Diaz R, Davalos G, Welsh LK, Portenier D, Guerron AD (2019) Use of magnets in gastrointestinal surgery. Surg Endosc 33(6):1721–1730
Gagner M. (2021) Laparoendoscopic magnetic gastrointestinal anastomosis. Chapter 14. In: Gagner M (ed) Magnetic Surgery. Springer, New York NY, 135–148.
Gagner M, Krinke T, LaPointe-Gagner M, Buchwald JN. (2023) Side-to-side duodeno-ileal magnetic compression anastomosis: design and feasibility of a novel device in a porcine model. Surg Endosc, epub ahead of print
Gagner M, Abuladze D, Koiava L, Buchwald JN, Van Sante N, Todd Krinke. (2023) First-in-human side-to-side magnetic compression duodeno-ileostomy with the Magnet Anastomosis System. Obes Surg, in submission
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al (2009) The Clavien-Dindo Classification of surgical complications: five-year experience. Ann Surg 250(2):187–196
Schlottmann F, Ryou M, Lautz D, Thompson CC, Buxhoeveden R (2021) Sutureless duodeno-ileal anastomosis with self-assembling magnets: safety and feasibility of a novel metabolic procedure. Obes Surg 31(9):4195–4202
Andalib A, Bouchard P, Alamri H, Bougie A, Demyttenaere S, Court O (2021) Single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S): short-term outcomes from a prospective cohort study. Surg Obes Relat Dis 17(2):414–424. https://doi.org/10.1016/j.soard.2020.09.015
Verhoeff K, Mocanu V, Zalasky A, Dang J, Kung JY, Switzer NJ, Birch DW, Karmali S (2022) Evaluation of metabolic outcomes following SADI-S: a systematic review and meta-analysis. Obes Surg 32(4):1049–1063. https://doi.org/10.1007/s11695-021-05824-w
Sánchez-Pernaute A, Herrera MÁR, Ferré NP, Rodríguez CS, Marcuello C, Pañella C et al (2022) Long-term results of single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S). Obes Surg 32(3):682–689. https://doi.org/10.1007/s11695-021-05879-9
Cottam D, Roslin M, Enochs P, Metz M, Portenier D, Smith D (2020) Single anastomosis duodenal switch: 1-year outcomes. Obes Surg 30:1504–1506
McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC (2015) Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg 102(5):462–479. https://doi.org/10.1002/bjs.9697
Reischl S, Wilhelm D, Friess H, Neumann PA (2021) Innovative approaches for induction of gastrointestinal anastomotic healing: an update on experimental and clinical aspects. Langenbecks Arch Surg 406(4):971–980. https://doi.org/10.1007/s00423-020-01957-1
Lee SW, Gregory D, Cool CL (2020) Clinical and economic burden of colorectal and bariatric anastomotic leaks. Surg Endosc 34(10):4374–4381. https://doi.org/10.1007/s00464-019-07210-1
Gagner M. (2021) Introduction: ideas and people leading to successful products for patient care leading to magnetic surgery. Chapter 1. In: Gagner M (ed) Magnetic Surgery. Springer, New York NY, 1–6.
Stepanov EA, Vasiliev GS, Nikolaev VV (1992) Treatment of intestinal fistulas in children by imposing a bypass anastomosis using magnetic devices. Surgery 11–12:93–97
Gatkin EJ, Razumovskij AJ, Korsunskij AA, Konovalov AK, Sergeev AV, Vinogradov AJ, Sein VA (2015) Interintestinal anastomoses formation using permanent magnet in surgical treatment of children with intestinal stomas. Z im NI Pirogova Khir. https://doi.org/10.17116/hirurgia2015545-50
Evans LL, Chen CS, Muensterer OJ, Sahlabadi M, Lovvorn HN, Novotny NM et al (2022) The novel application of an emerging device for salvage of primary repair in high-risk complex esophageal atresia. J Pediatr Surg 57(12):810–818. https://doi.org/10.1016/j.jpedsurg.2022.05.018
Kamada T, Ohdaira H, Takeuchi H, Takahashi J, Ito E, Suzuki N et al (2021) New technique for magnetic compression anastomosis without incision for gastrointestinal obstruction. J Am Coll Surg 232(2):170-177.e2
Hu B, Ye LS (2019) Endoscopic applications of magnets for the treatment of gastrointestinal diseases. World J Gastrointest Endosc 11(12):548–560
Funding
This study was supported by a research grant from GT Metabolic Solutions, Inc. (San Jose, CA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Michel Gagner is a consultant with stock options in GT Metabolic Solutions, and is a consultant for Medtronic, Inc. and Lexington Medical, Inc. with stock options in Lexington Medical, Inc. Guy-Bernard Cadière is a consultant for GT Metabolic Solutions and member of the scientific advisory board without remuneration. Andres Sanchez-Pernaute is a consultant and received fees from Novo Nordisk, Medtronic, Johnson & Johnson, and Gore Medical. David Abuladze is a consultant for GT Metabolic Solutions without remuneration. Todd Krinke is an employee with GT patents and stock options. Jane Buchwald is a GT consultant with GT stock options; she received grants from Ethicon, Inc., M.I.D., Society of Bariatric and Metabolic Surgeons of Kazakhstan, Medical Faculty of Mannheim, Holy Family Hospital, Israel, and the American College of Surgeons. Nathalie Van Sante was an employee of GT Metabolic Solutions, Inc. during the study and drafting of the manuscript. Lamees Almutlaq holds stock options in GT Metabolic Solutions. Antonio Torres is a consultant for, and has received a grant from GT Metabolic Solutions. Marc Van Gossum, Jana Dziakova, Levan Koiava, Maja Odovic, and Mathilde Poras, have no potential conflicts of interest and nothing to disclose.
Ethical approval
The study was conducted in compliance with the registered Clinicaltrials.gov protocol (#NCT05322122) and in accord with the ethical standards of the institutional research committee, and with the 1964 Helsinki declaration and its later amendments, as well as with ISO14155 regulations, 21 CFR Parts 11, 50, 54, 56, and 812 Good Clinical Practices.
Informed consent
Informed Consent was obtained with adequate understanding and consent of participating patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gagner, M., Cadiere, GB., Sanchez-Pernaute, A. et al. Side-to-side magnet anastomosis system duodeno-ileostomy with sleeve gastrectomy: early multi-center results. Surg Endosc 37, 6452–6463 (2023). https://doi.org/10.1007/s00464-023-10134-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10134-6