Abstract
Background
Laparoscopic cholecystectomy (LC) is the standard of care for benign gallstone disease. There are no robust Indian data on the 30-day morbidity and mortality of this procedure. A prospective multicentre observational study was conducted by the Indian Association of Gastro-Intestinal Endo Surgeons (IAGES) to assess the 30-day morbidity and mortality of LC in India.
Materials and methods
Participating surgeons were invited to submit data on all consecutive LCs for benign diseases performed between 09/12/2020 and 08/03/2021 in adults. Primary outcome measures were 30-day morbidity and mortality. Univariate and multivariate analyses were performed to identify variables significantly associated with primary outcomes.
Results
A total of 293 surgeons from 125 centres submitted data on 6666 patients. Of these, 71.7% (n = 4780) were elective. A total LC was carried out in 95% (n = 6331). Laparoscopic subtotal cholecystectomy was performed in 1.9% (n = 126) and the procedure were converted to open in 1.4% of patients. Bile duct injury was seen in 0.3% (n = 20). Overall, 30-day morbidity and mortality were 11.1% (n = 743) and 0.2% (n = 14), respectively. Nature of practice, ischemic heart disease, emergency surgery, postoperative intensive care, and postoperative hospital stay were independently associated with 30-day mortality. Age, weight, body mass index, duration of symptoms, nature of the practice, history of Coronavirus Disease-2019, previous major abdominal surgery, acute cholecystitis, use of electrosurgical or ultrasonic or bipolar energy for cystic artery control; use of polymer clips for cystic duct control; conversion to open surgery, subtotal cholecystectomy, simultaneous common bile duct exploration, mucocele, gangrenous gall bladder, dense adhesions, intraoperative cholangiogram, and use of drain were independently associated with 30-day morbidity.
Conclusion
LC has 30-day morbidity of 11.1%, 30-day mortality of 0.2%, conversion to open rate of 1.4%, and bile duct injury rate of 0.3% in India.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gallstone disease is a common clinical condition with rising prevalence [1]. Laparoscopic cholecystectomy (LC), the standard of care for gallstone disease and other benign gallbladder pathologies, is one of the most commonly performed surgical procedures worldwide [2]. A systematic review and meta-analysis [3] published in 2018 reported morbidity, bile duct injury, and mortality rates of this procedure at around 1.6–5.3%, 0.32–0.52%, and 0.08–0.15% respectively. This analysis, however, included data published over several decades and cannot be used to indicate the current safety profile of this procedure.
Although there are some single-centre retrospective studies from India reporting on the 30-day safety of LC [4], there is no large, prospective, multicentre study. Such data are needed for benchmarking, quality improvement, and informed patient consent. Even globally, there are very few large, prospective studies on 30-day morbidity and mortality of LC that use a validated method of capturing complications such as Clavien–Dindo grade [5]. Most studies on this topic are either retrospective (based on national registry data) or do not use a robust method of capturing complications [6,7,8].
This means there is globally an acute need for contemporary, prospective, multicentre, robust data on 30-day morbidity and mortality of LC. Because of these reasons, the Indian Association of Gastro-Intestinal Endo Surgeons (IAGES) decided to perform a prospective multicentre observational cohort study to evaluate the 30-day morbidity and mortality of LC in India.
Material and methods
Design
This was a multicentre, prospective, observational, cohort study. Google forms soliciting surgeons’ participation were circulated via social media and IAGES membership communication channels.
Patient eligibility
Participating surgeons submitted data on all consecutive LC performed by them between 09/12/2020 and 08/03/2021 in adult patients for benign gallbladder pathologies. 30-day follow-up data were collected for each patient.
Regulatory approval and data collection
Ethics committee permission was obtained at the leading primary Institute: (IRB: JLHL/IEC/2020), and the study was registered with the Clinical trial of Research of India (Registration number CTRI/2020/11/029448). Contributing Surgeons took part in a Zoom® meeting held to describe the study design and data capturing required for the study. Individual centres were recommended to follow local regulations and obtain additional permissions as needed. Anonymised patient data were collected using a Microsoft Excel® database as approved by the core team. Individual datasheets were carefully examined for errors, and any doubts were clarified with collaborators. Data were then pooled and analysed.
Inclusion and exclusion criteria
Inclusion criteria:
-
1.
Age ≥ 18 years
-
2.
LC as a primary procedure for benign symptomatic gallbladder disease
-
3.
LC for benign gallbladder disease performed with other procedures like hernia surgery, appendectomy, and hysterectomy was included in the study (but not the removal of a normal gallbladder as part of other surgery like Whipple's).
-
4.
Procedure performed in a centre in India
Exclusion criteria
-
1.
Patients undergoing cholecystectomy for malignancy
-
2.
Operations performed outside the study period
-
3.
Planned open cholecystectomy
Primary outcome
30-day morbidity and mortality of patients undergoing LC in India.
Secondary outcome
-
1.
To assess procedure-specific complication rates such as bleeding, bile leak, bowel injury, bile duct injury, and conversion to open surgery.
-
2.
To study the effect of a range of patient-specific, surgeon-specific, and facility-specific variables on 30-day morbidity and mortality.
Statistical analysis
Data were analysed using IBM SPSS version 26 and Jamovi version 1.8.4. Normality was tested using Kolmogorov Smirnoff test. A value of < 0.05 was considered statistically significant. Descriptive statistics were applied to numerical variables. Data were represented as mean ± SD (standard deviation) or median and IQR (interquartile range) as appropriate. Frequencies were compared using the Chi-Square test or Fisher's exact test as appropriate and means were compared using the t test. Binomial logistic regressions were carried out with 30-day morbidity (all Clavien-Dindo grades) or 30-day mortality as dependent variables to test if variables were independently associated with dependent outcomes. No attempt was made to develop a predictive model.
Results
Basic demographics
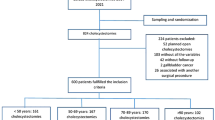
A total of 293 surgeons from 125 institutes contributed data on 6666 patients. Of these, 68.4% (n = 4544) were females with a mean age of 45.5 ± 14.8 years. The mean body mass index (BMI) was 26.08 ± 4.76 kg/m2. Approximately 43.1% of patients suffered from at least one other co-morbidity. Nearly 3.8% (n = 255) patients had a history of Coronavirus Disease-2019 (COVID-19) infection and 17.6% (n = 1174) of patients had undergone a previous open or laparoscopic major intraabdominal surgery. Most (78.3%) surgeons had > 10 years’ experience in laparoscopy; 12.3% had 6 to 10 years experience; 7.4% had 1–5 years experience; and 2.0% had < 1 year experience in laparoscopy. Data on 30-day morbidity and mortality were available for all the patients giving us a 100% follow-up in this study.
Indications, investigations, and prior ERCP/cholecystostomy
Majority (71.7%; n = 4780) of the procedures were elective. The indication for surgery was biliary colic in 65.4% and acute cholecystitis in 17.3%. A preoperative Ultrasound scan was performed on all the patients in this study. Ultrasound findings of increased wall thickness; impacted stone at neck; and pericholecystic fluid were seen in 30.9% (n = 2061), 11.3% (n = 756), and 16% (n = 1066), respectively. Magnetic resonance cholangiopancreatography (MRCP) was performed in 14% (n = 932) while a Computerised Tomography (CT) abdomen was carried out in 6.3% (n = 419). Approximately 7% (n = 463) patients had undergone a preoperative Endoscopic Retrograde Cholangio-Pancreatogram (ERCP) and 0.7% (n = 46) patients had a prior percutaneous cholecystostomy.
Surgical approach, important findings, and operating time
A total laparoscopic cholecystectomy was performed in 95% (n = 6331) of the patients while laparoscopic subtotal cholecystectomy was performed in 1.9% (n = 126). Conversion to open cholecystectomy was needed in 1.4% of patients (n = 96). A simultaneous laparoscopic common bile duct exploration was performed in 1.2% (n = 79) of patients. An additional procedure like appendectomy, hysterectomy, ureteric stone removal, or hernia repair was carried out in 0.5% (n = 34) patients. An intraoperative cholangiogram was performed in 1.4% (n = 95) patients. Cystic artery was controlled using titanium clips in 83.1% (n = 5537), polymer clips in 5.4% (n = 360), electrosurgical energy in 5.4% (n = 360), ultrasonic /bipolar energy in 3.7% (n = 252), and by ligation in 2.2% (n = 151). Cystic duct was controlled by titanium clips in 81.3% (n = 5412), polymer clips in 10.8% (n = 721), ligation in 4.3% (n = 292), and transfixation in 3.3% (n = 225). Mucocele, empyema, gangrene, and perforation were noted in 8.1% (n = 540), 8.7% (n = 581), 3.3% (n = 222) and 3.4% (n = 228), respectively. A “frozen” Calot’s triangle was encountered in 11.2% (n = 745). An intraabdominal drain was used in 29.3% (n = 1955). The mean duration of surgery was 53 ± 36 min, and the mean duration of postoperative stay was 1.85 ± 1.41 days.
Histopathology
Histopathology revealed chronic cholecystitis in 70.7% (n = 4177), acute cholecystitis in 20.1% (n = 1338), and xanthogranulomatous cholecystitis in 1.4% (n = 101). In approximately 0.5% (n = 30) patients, histology revealed an incidental malignancy.
Readmission/reintervention
Approximately 0.9% (n = 58) patients needed to be readmitted during the study period. Additional endoscopic, radiological, and surgical procedure were needed in 0.4% (n = 27), 0.5% (n = 35), and 0.3% (n = 19), respectively.
30-day morbidity and mortality
A 30-day Morbidity was reported in 11.1% (n = 743) and 14 (0.2%) patients died during the 30 days. Most of the complications were Clavien–Dindo grade I (2.8%) or grade II (7.2%). Grade III (0.9%), grade IV (0.2%), and grade V (0.2%) complications were less common (Table 1). Bleeding was encountered in 1.5% (n = 103), and it needed blood transfusion in 0.7% (n = 44). A bowel injury occurred in 0.1% (n = 4), and bile duct injury was seen in 0.3% (n = 20). Bile leak and intraabdominal collections were reported in 0.9% (n = 58) and 0.8% (n = 56), respectively. Wound and chest infections were noticed in 1.5% (n = 98) and 0.4% (n = 27), respectively. One patient each developed deep venous thrombosis and pulmonary embolism. Five patients (0.1%) had a myocardial infarction and 15 patients (0.2%) had a stroke. Approximately 8.5% (n = 568) needed intensive care postoperatively (Table 1).
Several variables were significantly associated with 30-day morbidity on univariate analysis (Tables 2 and 3). On binomial, logistic regression, only age, weight, BMI, duration of symptoms, nature of surgeon practice, history of COVID-19, previous major abdominal surgery, acute cholecystitis (vs biliary colic), use of unipolar electrosurgical energy or ultrasonic/bipolar energy for cystic artery control (vs titanium clips); use of polymer clips for cystic duct control (vs titanium clips); conversion to open surgery, subtotal cholecystectomy, simultaneous common bile duct exploration (vs laparoscopic cholecystectomy), mucocele, gangrenous gallbladder, dense adhesions, intraoperative cholangiogram, and use of abdominal drain were independently associated with 30-day morbidity (Table 4).
Similarly, many variables were associated with 30-day mortality on univariate analysis (Tables 5 and 6). But on binomial, logistic regression only nature of surgeon practice, ischemic heart disease, emergency surgery, postoperative ICU stay, and postoperative duration of stay were independently associated with 30-day mortality (Table 7).
Discussion
This large prospective, multicentre study of 30-day morbidity and mortality of LC in India conducted under the auspices of the Indian Association of Gastrointestinal Endoscopic Surgeons revealed 30-day morbidity of 11.1%, 30-day mortality of 0.2%, conversion to open surgery rate of 1.4%, and bile duct injury rate of 0.3%.
In comparison, Pucher et al. in their meta-analysis of 505,292 patients reported a conversion rate, BDI, morbidity, and mortality rates of 4.2–6.2%, 0.32–0.52%, 1.6–5.3%, and 0.08–0.14%, respectively [3]. But this meta-analysis included data from 150 studies performed over several decades, and authors admitted that data quality and reporting were heterogeneous with significant “reporting bias”. In comparison, ours is a prospective study with 100.0% follow-up. Moreover, because this meta-analysis included data from studies over a prolonged period, it cannot represent the contemporary picture. The Chole-S study is probably the only similar study in the literature [8]. It is a national (United Kingdom), prospective study on a similar number of patients. The 30-day morbidity rate in that study of 10.8% seems comparable to our 11.1%. Similarly, the 30-day mortality rate in that study was 0.12% compared to 0.2% in our study. Even that study was published six years ago.
In this study, we focussed on LC and not on the planned open procedure as LC has now become the standard of care for benign gallbladder disease even in developing countries such as India and the authors cannot think of any situation where they would “plan” to do an open cholecystectomy from the outset. Indeed, even though we had mentioned “planned” open cholecystectomy as an exclusion criterion for this study to avoid any confusion, none of our collaborators performed any planned open cholecystectomy during the study period.
Age was independently associated with 30-day morbidity in our study. Similar results have been noticed by other authors [9, 10]. This is probably due to increased frailty, presence of co-morbidities, higher use of antiplatelet and anticoagulants, and difficult local anatomy due to recurrent attacks and previous surgeries in these patients. Similarly, obesity, which was independently associated with 30-day morbidity in our study was associated with a higher risk of conversion to open surgery and bile duct injury in previous studies too [10,11,12]. This may be due to technical difficulties and obesity-associated co-morbidities. We found ischemic heart disease (IHD) to be independently associated with 30-day mortality. Others have also shown heart disease to be associated with a prolonged hospital stay, increased risk of readmissions, and resource utilisation in patients undergoing LC [13].
History of COVID-19 was noted in 3.8% of the patients and was independently associated with 30-day morbidity in our study. The countrywide lockdown for COVID-19 ended in India on 31st May 2020 and the gradual process of unlocking started on 1st June 2020. In most hospitals, elective surgeries had resumed by the case enrolment period of December 2020 to March 2021. This is further confirmed by the majority (71.7%; n = 4780) of the procedures being elective in our study. However, it is possible that the presentation of patients in this study was delayed due to the pandemic and this may have impacted the morbidity and mortality. But given that there is no robust pre-pandemic data on the outcomes of LC from India, one cannot be certain of that. Similarly, we are unable to deduce if patients with higher co-morbidity were excluded during the study period given the lack of pre-pandemic multicentre Indian data on this group of patients.
Acute cholecystitis and intraoperative findings of mucocele, gangrene, and dense adhesions were independently associated with 30-day morbidity in this study. These findings are unsurprising and others have also found acute cholecystitis to be associated with longer operating times, higher conversion rates, morbidity, and mortality [11, 12].
Emergency surgery, postoperative ICU stay, and prolonged hospital stay were also found to be independently associated with 30-day mortality in this study. Similar results have also been seen in other studies [11, 12, 14]. Emergency surgery for acute cholecystitis is associated with longer operating times, higher conversion rates, higher bile leak rates, and longer hospital stay [14]. At the same time, early laparoscopic cholecystectomy has shown to be safer than delayed surgery for acute cholecystitis in a recent Cochrane review [15]. There is no contradiction between the two observations as though at higher risk than patients without acute cholecystitis, early LC is still safer in this population than delayed LC.
The method of controlling cystic duct and artery was independently associated with 30-day morbidity in this study. Somewhat counterintuitively, the use of more secure locking polymer clips for cystic duct closure was associated with higher morbidity than the use of titanium clips. Similarly, the use of electrosurgical energy, ultrasonic energy, and advanced bipolar for cystic artery was associated with higher morbidity. These findings are probably due to selection bias as surgeons might have used these methods to control cystic duct and artery in more difficult cases. In a recently published review, the authors did not find any significant difference in outcomes between the different methods used to control the cystic duct [16]. There is probably hence a need for randomised studies to understand this better. Similarly, an Intraoperative cholangiogram was associated with higher 30-day morbidity—once again probably due to its selective use in difficult cases as only 1.5% of patients in this study underwent an intraoperative cholangiogram. This is probably also the explanation for drains being associated with higher morbidity.
Laparoscopy converted to open cholecystectomy, subtotal cholecystectomy, and simultaneous common bile duct explorations were all independently associated with 30-day morbidity in our study. These findings are unsurprising and have been confirmed by previous authors [17, 18].
A total of 34 patients in our study underwent additional surgeries such as hernia repair, appendectomy, ureteric stone removal, or hysterectomy. These combined surgeries offer the benefit of two procedures in single anaesthesia and hospital admission [19]. However, this advantage may come at a cost. We found that combining another procedure with LC was associated with higher 30-day morbidity on univariate analysis. More focussed studies need to examine this issue in more detail in the future.
Somewhat counterintuitively, we observed a lower risk of 30-day morbidity and mortality in the hands of surgeons with < 1 year of experience. This difference was not significant, and there can be several explanations for this observation—such as low numbers (n = 134) or that surgery was taken over by more experienced surgeons if found to be difficult intraoperatively as surgeons in this category would typically be trainees.
Limitations and strengths
The biggest weakness of this study is the self-reporting of complications. However, the authors believe anonymised data collection and pooling before analysis would have encouraged collaborators to submit accurate data as there was no personal incentive to underreport complications. And the morbidity and mortality rates in this study seem similar to what authors see in their institutions perhaps suggesting complications were fully reported. Other possible concerns could be regarding the inclusion of all consecutive cases during the study period. Though we repeatedly reminded our collaborators to include all consecutive cases during the study period to get rid of selection bias, we cannot be certain of this.
The study included surgeons practising in different setups (government hospitals as well as small and large private hospitals) with wide variation in health care facilities. The surgeons' expertise and training differed too as did the patient population thus leading to heterogenicity of data. Also, since ours was a multicentre study, the preoperative workup, and postoperative care, and overall quality of care would have varied from one institution to the other. But it was important for obtaining an estimate of the true picture of 30-day morbidity and mortality of LC in India. Although it was not our purpose to publish individual centre outcomes to preserve anonymity, surgeons in these centres should be able to compare their data to the pooled national data reported in this study and drive local quality improvement projects.
The use of Clavien–Dindo scoring has its limitations as it does not tell us anything about the nature of complications. To overcome this, we have also reported individual complications. Another potential weakness of our study is the 30-day follow-up. We would, therefore, not have been able to capture morbidity and mortality occurring after that period. The use of a 90-day follow-up [20] could overcome this weakness. At the same time, 30-day morbidity and mortality are widely used as surrogates for surgical safety in the academic literature.
At the same time, there are few prospective, contemporary, multicentre national studies on LC on such a large number of patients making this study a valuable addition to the surgical literature on this topic. It is also the first large experience from India with patients operated across the entire spectrum of Indian hospitals allowing us to obtain close to a true estimate of the safety of LC in India and also test for independent associations of variables with 30-day morbidity and mortality. Our use of validated CD grade of complications for capturing 30-day morbidity, 100% follow-up rate, and careful, manual checking of data are other factors worth highlighting.
Conclusion
This multicentre, prospective study of 6666 patients undergoing LC in 125 centres found a 30-day morbidity and mortality rate of 11.1% and 0.2%, respectively. Factors independently associated with 30-day mortality were the nature of surgeon practice, ischemic heart disease, emergency surgery, postoperative ICU stay, and postoperative duration of stay. Age, body weight, BMI, duration of symptoms, nature of surgeon practice, history of COVID-19, previous major abdominal surgery, acute cholecystitis (vs biliary colic), use of electrosurgical energy or ultrasonic/ advanced bipolar energy for cystic artery control (vs titanium clips); use of polymer clips for cystic duct control (vs titanium clips); conversion to open surgery, subtotal cholecystectomy, simultaneous common bile duct exploration (vs laparoscopic cholecystectomy), mucocele, gangrenous gallbladder, dense adhesions, intraoperative cholangiogram, and use of abdominal drain were independently associated with 30-day morbidity. Our results show that morbidity and mortality following LC in Indian hospitals are comparable to that in other large series reported from other parts of the world. We also encourage more surgeons from other Indian hospitals to participate in similar collaborative studies.
References
Stinton LM, Shaffer EA (2012) Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver 6(2):172–187
Lunevicius R, Nzenwa IC, Mesri M (2022) A nationwide analysis of gallbladder surgery in England between 2000 and 2019. Surgery 171(2):276–284
Pucher PH, Brunt LM, Davies N et al (2018) Outcome trends and safety measures after 30 years of laparoscopic cholecystectomy: a systematic review and pooled data analysis. Surg Endosc 32(5):2175–2183
Kaushik R, Sharma R, Batra R et al (2002) Laparoscopic cholecystectomy: an Indian experience of 1233 cases. J Laparoendosc Adv Surg Tech A 12(1):21–25
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Enochsson L, Thulin A, Osterberg J et al (2013) The Swedish Registry of Gallstone Surgery and Endoscopic Retrograde Cholangiopancreatography (GallRiks): a nationwide registry for quality assurance of gallstone surgery. JAMA Surg 148(5):471–478
Harboe KM, Bardram L (2011) Nationwide quality improvement of cholecystectomy: results from a national database. Int J Qual Health Care 23(5):565–573
CholeS Study Group, West Midlands Research Collaborative (2016) Population-based cohort study of outcomes following cholecystectomy for benign gallbladder diseases [published correction appears in Br J Surg. 2018 Aug;105(9):1222]. Br J Surg 103(12):1704–1715.
Sandblom G, Videhult P, Crona Guterstam Y et al (2015) Mortality after a cholecystectomy: a population-based study. HPB 17(3):239–243
Aziz H, Pandit V, Joseph B et al (2015) Age and Obesity are Independent Predictors of Bile Duct Injuries in Patients Undergoing Laparoscopic Cholecystectomy. World J Surg 39(7):1804–1808
Ibrahim S, Hean TK, Ho LS et al (2006) Risk factors for conversion to open surgery in patients undergoing laparoscopic cholecystectomy. World J Surg 30(9):1698–1704
Rosen M, Brody F, Ponsky J (2002) Predictive factors for conversion of laparoscopic cholecystectomy. Am J Surg 184(3):254–258
Boehme J, McKinley S, Michael Brunt L et al (2016) Patient comorbidities increase postoperative resource utilization after laparoscopic and open cholecystectomy. Surg Endosc 30(6):2217–2230
Kanakala V, Borowski DW, Pellen MG et al (2011) Risk factors in laparoscopic cholecystectomy: a multivariate analysis. Int J Surg 9(4):318–323
Gurusamy KS, Davidson C, Gluud C, et al. Early versus delayed laparoscopic cholecystectomy for people with acute cholecystitis. Cochrane Database Syst Rev 2013, Issue 6. Art. No.: CD005440.
van Dijk AH, van Roessel S, de Reuver PR et al (2018) Systematic review of cystic duct closure techniques in relation to prevention of bile duct leakage after laparoscopic cholecystectomy. World J Gastrointest Surg 10(6):57–69
Ji W, Li LT, Li JS (2006) Role of laparoscopic subtotal cholecystectomy in the treatment of complicated cholecystitis. Hepatobiliary Pancreat Dis Int 5(4):584–589
Ricci C, Pagano N, Taffurelli G et al (2018) Comparison of Efficacy and Safety of 4 Combinations of Laparoscopic and Intraoperative Techniques for Management of Gallstone Disease with Biliary Duct Calculi: A Systematic Review and Network Meta-analysis. JAMA Surg 153(7):e181167
Savita K, Khedkar I, Bhartia VK (2010) Combined procedures with laparoscopic cholecystectomy. Indian J Surg 72(5):377–380
Manuel-Vázquez A, Latorre-Fragua R, Ramiro-Pérez C et al (2017) Ninety-day readmissions after inpatient cholecystectomy: A 5-year analysis. World J Gastroenterol 23(16):2972–2977
Acknowledgements
The authors would like to thank the following: Dr. Ajay Thakker (Chairman and MD of Jupiter hospital where the primary investigation was carried out). Participating Investigators: Mrs. Minaxi Prajapati, Dr. Aisha Aga for technical assistance, data segregation, and maintenance throughout the study period. Dr. Paresh Koli (www.researchplexus.com) for statistical analysis. Dr. Ansari Amir, Dr. Dawle Anjali, Dr. Deshpande Aparna, Dr. Kavalakat Alfie, Dr. Khomane Gorakshanath, Dr. Mishra Shashank, Dr. Mulchandani Dheeraj, Dr. Muthukumar Gunasekaran, Dr. Mutreja Jagdish, Dr. Rajput H, Dr. Solanki Rashesh, Dr. Sonar Sanjay, and Dr. Suthar Ketankumar for taking part in the study. Collaborative Author list: A Karthik, Agarwal Lakshman, Agarwal Amit, Agarwalla Rishabh, Aggarwal Manas, Ahuja Anmol, AK Varadaraj, Akhtar Murtaza, Alinger Temsula, AnnaReddy Dinakar Reddy, Ayyar Srinivas, Badgoti Rambabu, Bagree Rajendra, Baig Sarfaraz, Baijal Manish, Bains Lovenish, Bairwa Banwari lal, Bali Rajandeep, Ballal Rajesh, Bandlur Sharath, Bansal Somendra, Bahadur Akshay, Bhagwat Sonali , Bhalla Bhavneet, Bhatt Jatin, Bhattacharjee Siddhartha, Bhojwani Rajesh, Bisht S D, Boddipalli Arjun, Bodra Pankaj, Borgaonkar Vijay, Boruah Prashanta, Brahma Rocket Chandra, Champawat Chitra, Chandak Kanhaiyya , Chandrasekar Sakthivel, Chatterjee Bitan, Chatterjee Shamita, Chaudhuri Tamonas, Chauhan Vikram, Chinnathambi Madeswaran, Chopra Shreya, Choudary Aditya, Choudhury Sourav, Choudhury Supriya, Chowbey Pradeep, Chowdhury A H, Dalal Ashwani, Dalal Usha, Dalvi Abhay, Das Chitta, Das Gunabhi Ram, Das Jayanta kumar, Datta Arupabha, Datta Rupjyoti, Deka Kunal, Dey Ashish, Dey Sumanta, Dhawan Monika, Doctor Nilesh, Donepudi B.Poornima, Dubey Sanjay, Easwaramoorthy S, Ekka Nishith, Eppa Vimalakar Reddy, Geyfane Naima, Goel Amitabh, Goel Apoorv, Goel Deep, Gowtham Thakut, Goyal Pankaj, Gupta Achal, Gupta Rajkumar, Gupta Rahul, Gupta Shalu, Gupta Shardool, HM Lokesh, Hamdani Nisar, Haridas Sarath, Hazarika Bhaskar, Heer Vikas, Hiremath Srikantaiah, I Hariharasaran, Ibrarullah Mohammad, Islam Chaidul, Islam Samsul, Ismail Mohammed, Jain Amit, Jain Mohit, Jain Parakash, Jain Sumita, Jathar Advait, Jassi Nikita, Jankar Samrat, Jeese James, Jindal Yashpaul, Joshi Abhijit, Joshi Praveen, Joy Rejana, K Pooja, K Prasad, K Anirudhan, Kalikar Vishakha, Kondeti Adityakalyan, Kamat Manmohan, Kapoor Abhimanyu, Kashmira Mayank, Katta Rohan, Kaur Jaspreet, Khan Hosni, Khanduri Archana, Khanna Ajay, Khandelwal RG, khanna Subhash, Khanna Shashi, Khiangte Elbert, Khullar Rajesh, Khuroo Suhail, kishore Shashank, Konwar Uttam, Kothari Shyam, Kothari Chaitanya Prakash, Kulkarni Jyotsna, Kumar Anil, Kumar Bhartendu, Kumar Durgesh, Kumar Jitendra, Kumar Shashidhar, Kumar Saurabh, Kumar Kshitiz, Kumar Puneet, Kumaran Ranjith, Kynjing Hampher, Lakshman Krishnaswamy, Lakshmi Suchitra, Lakshmi Kona, Lakshmikantha Nishanth, Lal Pawan, Lalhruaizela Samuel, Lepcha Alfred, Litake Manjusha, Lobo Lancelot, Lohiya Sushil, Longkume Temsutoshi, M MuniReddy, M Vijaykumar, Madhu Sivakumar, Mahadik Deepak, Malhotra Manan Singh, Mallipudi BV Prasad, Malviya Nishant Kumar, Mandal Suman, Manek Parth, Manglik Shresth, Mohd Faiz, Mathur Alok, Medappil Noushif, Meher Sadananda, Mehrotra Magan, Mehta Diksha, Meenakshisundaram Senthil, MG Prakash, Mishra Lalan, Mishra Subhash, Mithi Taher, Mittal Tarun, Mittal kushal, Madan Rajan, Modi Abhiram, Momin Erbaz, Mohan Rajashekar, Mulpuri Ramya, Muqueem Khalid, Murchite Sheetal A, Mushtaque Majid, N Dileep, Nagakumar Nikhil, Naik N Ramprasanna, Naik Madhavi, Nagar Anand, Nandakumar Govind, Nara Bharat Kumar, Nath Barun, Nayak Darshan, Nayak Manjunath, Niranjan Rohit, Ninan Oommen Ashok, Om Prabha, Panchauli Aashutosh, Paramashivaiah Niranjan, Pahari Hirak, Parikh Chirag, Patankar Roy, Patel Rakeshkumar, Patel Danesh, Patel Deepak, Patel Tejas, Patani Tanmaye, Paul Soumen, Paul Pratik, Poddar Anshuman, Porwal Pankaj, Prakash Anand, Prasad Arun, Priya Pallawi, Priyadarshan Gaurav, Puri Puneet, R Durai, R Santhosh, Rahate Prashant, Rahman Mohsinur, Rajgopal Mahesh, Ramesh B S, Rangad Gordon, Rao Prashanth, Rashid Arshad, Ray Sandip, Ray Udipta, Rege Sameer, Rengan Shyam, Rupavath Rajendar, S Anand, S ArunKumar, S DineshKumar, S Viswanath, Shah Amit, Sahadevan Sajeesh, Sangade Vishal, Saraswat Anurag, Sarkar Sauradeep, Sarwal Ankush, Saha Snehasish, Shah Harsh, Shah Shrenik, Sharma Anil, Sharma Abadhesh, Sharma Meenakshi, Sharma Varsha, Shellagi Nikhil, Sheth Harsh, Shetty Pravin, Shetty Sanjeev Vikram, Shrimal Ankur, Shrinivasan Pranav, Singh Arvind, Singh Abhishek, Singh Abhiyutthan, Singh Chandrapal, Singh Charan, Singh Gurbhaij, Singh Gurbachan, Singh Saurav , Singh Harmanmeet, Singh Shailendra Pal, Sinha Nawneet Kumar, Somani Aalok, Soni Vandana, Srivastava Sanjai, Surapaneni Sushama , Suryawanshi Pravin, Tantia Om, Tauheed Fahad, Thangavelu Ashwin, Thota Anuroop, Tiwari Abhishek, Tiwary Satyendra K, Tripathi Pradeep, Umapathi Lohith, Varshney Peeyush, Vashistha Ashish, Vats Ravindra, Verma Ram Kumar, Verma Arunima, Vyas Soumil, Wani Ajaz, Wani Sachin, Yadav Amit, Yadav Anand Kumar, Yerraguntla Raghu, Yaseen Mohammed.
Funding
Funded by the Indian Association of Gastro-Intestinal Endo Surgeons (IAGES).
Author information
Authors and Affiliations
Consortia
Contributions
All authors have contributed in drafting the manuscript.
Corresponding author
Ethics declarations
Disclosures
Vinaykumar B. Thapar, Pinky M. Thapar; Ramen Goel, Ramesh Agarwalla, Prashant H. Salvi, Amrit M. Nasta, and Kamal Mahawar have no conflict of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Collaborative author list has been included in ‘Acknowledgements’ section.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thapar, V.B., Thapar, P.M., Goel, R. et al. Evaluation of 30-day morbidity and mortality of laparoscopic cholecystectomy: a multicenter prospective observational Indian Association of Gastrointestinal Endoscopic Surgeons (IAGES) Study. Surg Endosc 37, 2611–2625 (2023). https://doi.org/10.1007/s00464-022-09659-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09659-z