Abstract
Background
Postoperative pancreatic fistula (POPF) and postoperative fluid collection (POFC) are common complications after distal pancreatectomy (DP). The previous method of reducing the risk of POPF was the application of a polyglycolic acid (PGA) sheet to the pancreatic stump after cutting the pancreas with a stapler (After-stapling); the new method involves wrapping the pancreatic resection line with a PGA sheet before stapling (Before-stapling). The study aimed to compare the incidence of POPF and POFC between two methods.
Methods
Data of patients who underwent open or laparoscopic DPs by a single surgeon from October 2010 to February 2020 in a tertiary referral hospital were retrospectively analyzed. POPF was defined according to the updated International Study Group of Pancreatic Fistula criteria. POFC was measured by postoperative computed tomography (CT).
Results
Altogether, 182 patients were enrolled (After-stapling group, n = 138; Before-stapling group, n = 44). Clinicopathologic and intraoperative findings between the two groups were similar. Clinically relevant POPF rates were similar between both groups (4.3% vs. 4.5%, p = 0.989). POFC was significantly lesser in the Before-stapling group on postoperative day 7 (p < 0.001).
Conclusions
Wrapping the pancreas with PGA sheet before stapling was a simple and effective way to reduce POFC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Although the morbidity and mortality rates after pancreatic surgery have decreased significantly in recent years due to the development of surgical techniques and improved perioperative management, postoperative pancreatic fistula (POPF) is still the most common and serious complication that cannot be easily resolved [1]. POPF after distal pancreatectomy (DP) is not as severe as that after pancreatoduodenectomy (PD); however, it is associated with other complications, including intra-abdominal abscess, intra-abdominal hemorrhage, delayed gastric emptying, and sepsis [2, 3]. Postoperative fluid collection (POFC) is also frequently observed after DP.
Several studies have suggested ways to reduce the incidence of POPF after DP, including hand-sewn closure, main-duct isolated ligation, transection, and closure using a stapling device, pancreatic transection using energy devices, fibrin sealants, and the use of patches or sheets [1, 4,5,6,7,8,9]. Despite much effort, the clinically relevant POPF (CR-POPF) rate after DP remains between 4 and 40%. Recently, as minimally invasive surgery has increased when performing DP in pancreatic cancer as well as benign to borderline tumors of the pancreas, it seems that cutting the pancreas using a stapler is one of the most commonly used methods [10]. In addition, Jang et al. introduced a simple method in their randomized clinical trial to reduce the risk of POPF by applying a polyglycolic acid sheet (PGA, Neoveil®, Gunze, Japan) to the pancreatic stump after cutting the pancreas using a stapler [11]. Neoveil® is a bio-absorbable (within 15 weeks) reinforcement material made from PGA.
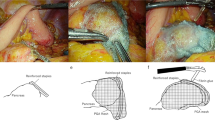
In the past, we also attached a PGA sheet on the pancreatic stump after cutting the pancreas with a stapler (After-stapling method), but recently, we have used the technique of wrapping the pancreas with a PGA sheet first and then stapling on it (Before-stapling method) (Fig. 1). Since there are no studies to evaluate the efficacy of reducing POPF and POFC according to the time of applying PGA sheet, this study aimed to compare the incidence of POPF and POFC between the After-stapling and Before-stapling methods.
Two different methods of applying a polyglycolic acid sheet (PGA, Neoveil®) to the pancreatic stump. In the After-stapling group (A–C), the PGA sheet was attached on the pancreatic stump after cutting the pancreas with a stapler. However, in the Before-stapling group (D–F), the pancreas was first wrapped with a PGA sheet before stapling, and then the pancreas was cut using a stapler
Methods
Study population and surgical procedure
This retrospective study comprised patients who underwent open or laparoscopic DP by a single surgeon from October 2010 to February 2020 in a tertiary referral hospital. Case report forms were used to record the patients’ demographic characteristics (age, sex, body mass index [BMI], pathologic diagnosis, operative method, spleen preservation, operative time, intraoperative blood loss, postoperative hospital stay duration, and postoperative complications). All consecutive cases of DPs were included in a prospectively recorded database. The pancreas was only transected using an Endo-GIA™ (endovascular gastrointestinal anastomosis stapler, Covidien, North Haven, CT, USA) stapler in both open and laparoscopic surgeries. The thickness and length of the cartridge of the Endo-GIA were selected by the surgeon, with consideration of the thickness, length, and texture of the pancreas. The fibrin sealants were applied to the resection margin of the pancreas in all patients. The patients were classified into the following two groups according to when the PGA sheet was applied: the After-stapling group, in which the PGA sheet was attached on the pancreatic stump after cutting the pancreas with a stapler, and the Before-stapling group, in which the pancreas was first wrapped with a PGA sheet, and then the pancreas was cut using a stapler (Fig. 1). A closed suction silicon drain was placed near the pancreatic stump. The amount of fluid in the closed suction drain was checked daily. The amylase and lipase levels in the serum and intraperitoneal fluid obtained from a closed drain were regularly checked. This study was approved by the Institutional Review Board of Yonsei University College of Medicine (approval number: 4-2020-0393).
Definition of variables and postoperative follow-up
POPF was defined according the updated International Study Group of Pancreatic Fistula (ISGPF) criteria established in 2016 that was retrospectively applied to all patients who underwent DP before 2016 [12]. A routine abdominal computed tomography (CT) was performed between postoperative days (PODs) 5 and 7 during hospitalization (short-term imaging follow-up). The first visit to the out-patient clinic after discharge was made between 1 and 2 weeks after discharge, at which time a basic medical interview was held. Moreover, the routine examinations, including blood testing and abdominal CT, were performed on POD 90. The next regular follow-up evaluations in the out-patient clinic were performed every 3–6 months to monitor for loco-regional or systemic recurrence in patients with a borderline tumor or ductal adenocarcinoma of the pancreas. For the patients with chronic pancreatitis, routine laboratory tests and abdominal CT were performed to check the remnant pancreas for recurrence of pancreatic cancer. To compare the difference in POFC between the After-stapling and Before-stapling groups, POFC was measured by CT performed on PODs 5–7 and 90. The hepatic venous phase with 3-mm thickness contrast-enhanced axial and coronal images was reviewed using a picture archiving and communication system (PACS) workstation (Marosis M-view 5.4; Marotech, Seoul, South Korea) at the pancreatic resection margin. Among the multiple cut images of the postoperative CT, the image showing maximum fluid collection was selected. The length and width on the axial image and the height on the coronal image were measured. The volume of POFC was calculated by multiplying the length, width, and height (Fig. 2).
Measurement of the postoperative fluid collection on a computed tomography (CT) image. Among the multiple cut images of the postoperative CT scan, the image with the largest fluid collection was selected. The length (a) and width (b) on the axial view (A) and the height (c) on the coronal view (B) were measured. The volume of the postoperative fluid collection was calculated by multiplying the length, width, and height (volume of fluid collection = a × b × c, mm3)
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation (SD). Differences in continuous variables between the two groups were tested using the Student’s t test. Categorical variables are expressed as number (percentage). The associations among different categorical variables were determined using the Chi-squared or Fisher’s exact test. Statistical significance was defined as p < 0.05. Statistical analyses were performed using SPSS 24.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
No differences in clinicopathologic findings and intraoperative outcomes
Of the 193 patients who underwent DP during the study period, 11 patients were excluded due to combined gastrointestinal surgery (stomach cancer, 6; colon cancer, 5); finally, 182 cases were enrolled in this study. Table 1 presents the clinicopathologic characteristics and intraoperative findings of the enrolled patients. There were 84 men and 98 women with a mean age of 58.3 ± 14.6 (range 17–86) years. The After- and Before-stapling groups comprised 138 and 44 patients, respectively.
There were no differences in age, sex, and BMI between the two groups. The most common pathologic diagnosis was pancreatic ductal adenocarcinoma (37.4%), followed by intraductal papillary mucinous neoplasm (13.7%), solid pseudo-papillary tumor (12.6%), and neuroendocrine tumor (9.9%). There was no difference in the pathologic diagnosis between the two groups (p = 0.364). The surgical methods, classified as open, laparoscopic, and laparoscopic attempted surgeries, were not different between the two groups (p = 0.426). There were no differences in the frequency of spleen preservation (p = 0.120), location of pancreatic resection (p = 0.423), operative time (p = 0.456), and intraoperative blood loss (p = 0.982) between the two groups (Table 1).
No differences in POPF rates and postoperative outcomes
Biochemical leakage occurred in 101 patients (55.5%). Eight patients (4.4%) had grade B POPF, but none had grade C POPF. According to the POPF grade, there was no difference in the POPF rates between the After-stapling group and Before-stapling group (biochemical leakage, 55.8% vs. 54.5%; grade B, 4.3% vs. 4.5%; p = 0.989). There were no differences in the volumes of fluid drained, according to the postoperative day (p = 0.430, p = 0.895, p = 0.428, p = 0.405), drain removal day (p = 0.749), and postoperative hospital stay (p = 0.614) between the two groups (Table 2).
Reduced POFC in the before-stapling group
POFC was observed in most patients (n = 169, 92.9%) on CT performed on POD 7. A new percutaneous drainage catheter was inserted in 4 patients of the After-stapling group because of ineffective drainage and increase in fluid collection volume during hospitalization. Even though none of the patients in the Before-stapling group received therapeutic interventions for POFC, there was no statistical difference in the rate of re-intervention between the two groups (2.9% vs. 0%, p = 0.574) (Table 2). Although the incidence of POFC on POD 7 did not differ between the two groups, the volume of POFC was significantly lower in the Before-stapling group than in the After-stapling group (37,423.2 ± 67,357.7 vs. 8,044.1 ± 9,011.7 mm3, p < 0.001). Even though POFC remained until POD 90 in approximately half of the patients (83, 45.6%), spontaneous regression was observed in all patients during the long-term follow-up period. There was no difference in the volume of POFC on POD 90 between the two groups. (p = 0.664) (Table 2). Twelve patients (10.1%) in the After-stapling group and 5 patients (11.4%) in Before-stapling group were readmitted to receive conservative management for their symptoms (abdominal pain, indigestion, fever, and wound problems). No patient was readmitted for re-intervention to treat POPF or POFC, and there was no difference in the readmission rate between the two groups (p = 0.782) (Table 2).
Discussion
The current study proposed a method to minimize the incidence of POPF or POFC after DP. This study showed that POFC can be reduced more effectively by making simple changes to the method of attaching a PGA sheet to the pancreatic stump. Although the CR-POPF rate was not significantly different between the After- and Before-stapling groups, the incidence of POFC was further reduced when the pancreas was first wrapped with a PGA sheet before stapling, and then stapling on it to cut the pancreas. In terms of POPF, although no difference was observed in the CR-POPF rate between the technique of applying the PGA sheet before and after stapling (4.3% vs. 4.5%, p = 0.989), the CR-POPF rate in both groups was sufficiently low, which was comparable to the rates reported by other studies that applied the PGA sheet to the pancreatic stump.
Despite various efforts to reduce the CR-POPF rate after DP, the CR-POPF rate remains between 4 and 40%, and it is still unclear which method is the most effective for reducing the POPF rate [1, 4,5,6,7,8,9]. Using the PGA sheet is one of the effective methods to reduce the incidence of POPF, with the CR-POPF rate being approximately 4% in other studies applying the PGA sheet to the pancreatic stump after DP [5, 9]. However, Jang et al. reported, in their recent multicenter randomized clinical trial, that the CR-POPF rate was 11.4% in PGA sheet wrapping group [11]. In our study, only grade B POPF occurred in 8 patients (4.4%) out of the 182 patients studied, and there was no patient with grade C POPF; our results either did not differ or were better than those of other previous studies [1, 4,5,6,7,8,9]. Based on these results, regardless of whether the PGA sheet is attached to the pancreatic stump after stapling (After-stapling group) or the pancreas is transected with a stapler after wrapping the pancreas with the PGA sheet (Before-stapling group), it can be concluded that using the PGA sheet is effective in reducing the incidence of POPF.
Given that the tearing of the pancreatic parenchyma is related to POPF on the pancreatic stump, a few methods, such as reducing the closing speed of the stapler [13,14,15,16] or using a stapling device with a pre-attached PGA sheet [17], were introduced to reduce the risk of injury to the pancreatic parenchyma. Other studies have demonstrated that prolonged pre-firing compression for approximately 3–5 min with a stapler can help to minimize the risk of POPF by decreasing the pancreatic thickness sufficiently to effectively stapling the pancreas [14,15,16]. We routinely performed pre-firing compression for 3–5 min before stapling in all patients and also performed the incision slowly for 1–2 min during firing.
In our literature review, we found a few studies that utilized the same method as ours. Ochiai et al. [9] first described the PGA wrapping method with GIA. Their procedure was the same as our method; during DP, the PGA sheet was wrapped around the predictive staple line of the pancreas to reduce compression tissue damage by a stapler device before stapling. In some cases, they used the Echelon 60 with the PGA sheet stocking type, which was released in only some countries and was not available in Korea. According to their results, severe POPF occurred in 10 out of 37 (27%) patients in the group that did not use PGA, whereas, in the group that used PGA, the incidence of severe POPF was only 1 out of 26 (4%) patients, showing a statistical difference (p = 0.017). Yamashita et al. also introduced the linear stapling device with a pre-attached PGA sheet (Endo-GIA™ Reinforced Reload with Tri-Staple™; black reload; 60-mm long; Covidien Japan Inc. Tokyo, Japan). They reported that the grade B or C POPF in the PGA sheet (+) group occurred in only one patient (5%) among the 22 patients, with the rate being significantly lower compared to that of the PGA sheet (−) group (5% vs. 30%, p = 0.0216) [17]. Considering some possible mechanisms that PGA sheet can reduce the incidence of POPF or POFC, first, when the pancreas is wrapped with a PGA sheet and then stapling is performed, the main pancreatic duct and branch duct in the pancreatic parenchyma at the pancreatic stump could be more completely sealed by closing the narrow gap between the staple and the pancreatic parenchyma. The second possible mechanism is that wrapping the pancreas with a PGA sheet before stapling can reduce the crushing injury to the pancreatic parenchyma when closing the jaw of the stapler. However, it is still unclear why the amount of POFC was lower in the Before-stapling group, even though the rates of biochemical leakage and CR-POPF were similar in both groups. One possible mechanism is that the PGA sheet inserted into the pancreas parenchyma with the stapler cartridge in the Before-stapling group could help heal the torn part of the pancreatic stump. POPF was defined as a peritoneal amylase value > 3 times the normal upper limit on POD 3; however, abdominal CT was performed between PODs 5 and 7. During PODs 2 or 4, the healing process might be more effective due to the PGA sheet embedded in the parenchyma of the pancreas. However, further studies, including animal experiments, are necessary to clarify the mechanism. To prevent crushing injuries, it is also important to select the proper Endo-GIA cartridge according to the pancreatic thickness and texture. If the pancreatic tissue is thick, a higher cartridge should be selected [18]. Apart from selecting an appropriate cartridge according to the thickness of the pancreas, the texture of the pancreatic tissue also needs to be evaluated; if the texture of the pancreatic tissue is firm, it may result in a crushing injury at the resected line of the pancreas. When the pancreas is wrapped with a PGA sheet, such crushing injury can be reduced.
In addition to the method of attaching the PGA sheet to the cut surface of the pancreas, other methods for reinforcement of the resection line of the pancreas have been also introduced. Hamilton et al. reported a study comparing the CR-POPF rate between patients who underwent stapled transection of the pancreas alone and patients who underwent stapled transection with reinforcement using commercially available mesh devices (Seamguard, W. L. Gore, Flagstaff, AZ, or Peristrips Dry, Synovis, St Paul, MN) through a randomized controlled trial [19]. The CR-POPF rate was significantly lower in the mesh reinforcement group than in the staple alone group (1.9% vs. 20%, p = 0.0007). Hassenpflug et al. investigated the benefit of covering the resection margin by a teres ligament patch [20]. In their study, although the overall CR-POPF rate was not reduced by the covering method (32.9% vs. 22.4%, p = 0.200), the coverage of the pancreatic remnant after DP was associated with less morbidities. Akca et al. introduced a method of covering the resection margin after DP with the gastric wall or omentum majus [21]. The no-cover group showed a higher frequency of POPF than the cover group did (22% vs. 10%, p > 0.05). In a prospective multicenter randomized controlled study, Park et al. utilized TachoSil (a biological sealing patch, Nycomed GmbH, Linz, Austria) to prevent the occurrence of POPF after DP [8]. There was no difference in the overall incidence of CR-POPF between the control group and TachoSil group (28.3% vs. 22.9%, p = 0.536).
This study has several limitations. First, this is a retrospective study and not a randomized controlled trial. Given that the method of wrapping the PGA sheet on the pancreas first and then stapling is a newly attempted technique in our clinical practice since November 2018, the changes in the surgical procedures over time should be considered. However, since all cases were performed by one surgeon, the selection of Endo-GIA cartridge according to the pancreatic thickness and texture and the type and location of closed suction drains inserted into the surgical field were applied equally to all patients. Given that these factors were important when assessing the presence of POPF or POFC, we were able to minimize the procedure-related bias. Second, we did not accurately measure the volume of fluids that actually accumulated in the abdominal cavity. For the measurement of fluid volume, the length and width on the axial CT image and the height on the coronal CT image were measured as a hexahedral value; thus, it does not completely represent the actual volume of fluid in a real irregular shape. If volumetry was used, the exact fluid volume could have been measured, but since the same measurement method was applied to all patients, the comparison of fluid volumes between the two groups is appropriate. Third, even though the amount of POFC on POD 7 was lower in the Before-stapling group than in the After-stapling group, there were no statistical differences in the rates of re-intervention or the natural course of spontaneous resolution of asymptomatic POFC between the two groups. Based on these results, this study failed to show advantages of the Before-stapling technique other than the lower amount of POFC. Fourth, this study enrolled a relatively small number of patients. Although 44 patients were enrolled in this study, which was more than the number of patients enrolled in previous studies (22 [17] and 26 [9] patients) using a similar method, it is still a small number of patients. Lastly, the results of this study are difficult to generalize, since all surgical procedures were performed by a single surgeon. Further research is needed in the future to prove the feasibility and effectiveness of Before-stapling method with randomized control trial in large patients group.
In conclusion, applying a PGA sheet to the resection line before or after stapling during DP was an effective method to reduce the incidence of CR-POPF, although no difference was observed between the Before- and After-stapling groups. Even though most cases of fluid accumulation occurring in the surgical field after DP were of no clinical significance, applying a PGA sheet before stapling was a simple and effective way to reduce the risk of fluid collection on the pancreatic stump.
References
Miyasaka Y, Mori Y, Nakata K, Ohtsuka T, Nakamura M (2017) Attempts to prevent postoperative pancreatic fistula after distal pancreatectomy. Surg Today 47(4):416–424
Strasberg SM, Linehan DC, Clavien P-A, Barkun JS (2007) Proposal for definition and severity grading of pancreatic anastomosis failure and pancreatic occlusion failure. Surgery 141(4):420–426
Butturini G, Marcucci S, Molinari E, Mascetta G, Landoni L, Crippa S, Bassi C (2006) Complications after pancreaticoduodenectomy: the problem of current definitions. J Hepatobiliary Pancreat Surg 13(3):207–211
Bilimoria MM, Cormier JN, Mun Y, Lee JE, Evans DB, Pisters PW (2003) Pancreatic leak after left pancreatectomy is reduced following main pancreatic duct ligation. Br J Surg 90(2):190–196
Yamamoto M, Hayashi MS, Nguyen NT, Nguyen TD, McCloud S, Imagawa DK (2009) Use of Seamguard to prevent pancreatic leak following distal pancreatectomy. Arch Surg 144(10):894–899
Pulvirenti A, Landoni L, Borin A, De Pastena M, Fontana M, Pea A, Esposito A, Casetti L, Tuveri M, Paiella S, Marchegiani G, Malleo G, Salvia R, Bassi C (2019) Reinforced stapler versus ultrasonic dissector for pancreatic transection and stump closure for distal pancreatectomy: a propensity matched analysis. Surgery 166(3):271–276
Suzuki Y, Kuroda Y, Morita A, Fujino Y, Tanioka Y, Kawamura T, Saitoh Y (1995) Fibrin glue sealing for the prevention of pancreatic fistulas following distal pancreatectomy. Arch Surg 130(9):952–955
Park JS, Lee DH, Jang JY, Han Y, Yoon DS, Kim JK, Han HS, Yoon Y, Hwang D, Kang CM, Hwang HK, Lee WJ, Heo J, Chang YR, Kang MJ, Shin YC, Chang J, Kim H, Jung W, Kim SW (2016) Use of TachoSil(®) patches to prevent pancreatic leaks after distal pancreatectomy: a prospective, multicenter, randomized controlled study. J Hepatobiliary Pancreat Sci 23(2):110–117
Ochiai T, Sonoyama T, Soga K, Inoue K, Ikoma H, Shiozaki A, Kuriu Y, Kubota T, Nakanishi M, Kikuchi S, Ichikawa D, Fujiwara H, Sakakura C, Okamoto K, Kokuba Y, Otsuji E (2010) Application of polyethylene glycolic acid felt with fibrin sealant to prevent postoperative pancreatic fistula in pancreatic surgery. J Gastrointest Surg 14(5):884–890
Zhang H, Zhu F, Shen M, Tian R, Shi CJ, Wang X, Jiang JX, Hu J, Wang M, Qin RY (2015) Systematic review and meta-analysis comparing three techniques for pancreatic remnant closure following distal pancreatectomy. Br J Surg 102(1):4–15
Jang JY, Shin YC, Han Y, Park JS, Han HS, Hwang HK, Yoon DS, Kim JK, Yoon YS, Hwang DW, Kang CM, Lee WJ, Heo JS, Kang MJ, Chang YR, Chang J, Jung W, Kim SW (2017) Effect of polyglycolic acid mesh for prevention of pancreatic fistula following distal pancreatectomy: a randomized clinical trial. JAMA Surg 152(2):150–155
Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M (2017) The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 161(3):584–591
Chikamoto A, Hashimoto D, Ikuta Y, Tsuji A, Abe S, Hayashi H, Imai K, Nitta H, Ishiko T, Watanabe M, Beppu T, Baba H (2014) Effects of the closing speed of stapler jaws on bovine pancreases. Surg Endosc 28(1):336–340
Okano K, Kakinoki K, Suto H, Oshima M, Maeda N, Kashiwagi H, Yamamoto N, Akamoto S, Fujiwara M, Takama H, Hagiike M, Usuki H, Suzuki Y (2010) Slow parenchymal flattening technique for distal pancreatectomy using an endopath stapler: simple and safe technical management. Hepatogastroenterology 57(102–103):1309–1313
Nakamura M, Ueda J, Kohno H, Aly MY, Takahata S, Shimizu S, Tanaka M (2011) Prolonged peri-firing compression with a linear stapler prevents pancreatic fistula in laparoscopic distal pancreatectomy. Surg Endosc 25(3):867–871
Hirashita T, Ohta M, Yada K, Tada K, Saga K, Takayama H, Endo Y, Uchida H, Iwashita Y, Inomata M (2018) Effect of pre-firing compression on the prevention of pancreatic fistula in distal pancreatectomy. Am J Surg 216(3):506–510
Yamashita YI, Tsujita E, Chikamoto A, Imai K, Kaida T, Yamao T, Umezaki N, Nakagawa S, Hashimoto D, Baba H (2017) Linear stapling device with pre-attached bioabsorbable polyglycolic acid felt reduces postoperative pancreatic fistula after distal pancreatectomy. Anticancer Res 37(4):1865–1868
Kim H, Jang JY, Son D, Lee S, Han Y, Shin YC, Kim JR, Kwon W, Kim SW (2016) Optimal stapler cartridge selection according to the thickness of the pancreas in distal pancreatectomy. Medicine (Baltimore) 95(35):e4441
Hamilton NA, Porembka MR, Johnston FM, Gao F, Strasberg SM, Linehan DC, Hawkins WG (2012) Mesh reinforcement of pancreatic transection decreases incidence of pancreatic occlusion failure for left pancreatectomy: a single-blinded, randomized controlled trial. Ann Surg 255(6):1037–1042
Hassenpflug M, Hinz U, Strobel O, Volpert J, Knebel P, Diener MK, Doerr-Harim C, Werner J, Hackert T, Büchler MW (2016) Teres ligament patch reduces relevant morbidity after distal pancreatectomy (the DISCOVER randomized controlled trial). Ann Surg 264(5):723–730
Akca A, Goretzki PE, Wirowski D, Renter MA, Bölke E, Matuschek C, Gerber PA, Lammers BJ (2013) Is the covering of the resection margin after distal pancreatectomy advantageous? Eur J Med Res 18(1):33
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Ji Su Kim, Seoung Yoon Rho, Dong Min Shin, Munseok Choi, Chang Moo Kang, Woo Jung Lee, and Ho Kyoung Hwang have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, J.S., Rho, S.Y., Shin, D.M. et al. Wrapping the pancreas with a polyglycolic acid sheet before stapling reduces the risk of fluid collection on the pancreatic stump after distal pancreatectomy. Surg Endosc 36, 1191–1198 (2022). https://doi.org/10.1007/s00464-021-08387-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08387-0