Abstract
Background
Adequate bowel preparation is a crucial step in colonoscopy procedure and has been identified as the cornerstone of a quality colonoscopy. Polyethylene glycol (PEG) for bowel cleansing still had up to 10 % unprepared colon.
Aim
We herein compare efficacy, acceptability, tolerance and safety of sodium phosphate (NaP) tablets and split-dose PEG for bowel cleansing.
Patients and methods
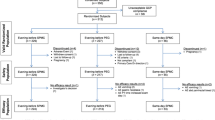
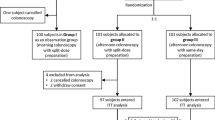
A prospective non-inferiority randomized trial was performed and registered on www.clinicaltrials.gov (NCT01840553). Patients were randomized to either 32 NaP tablets or 4 L of PEG. Blind readers assessed the efficacy of colon cleansing using the Boston Bowel Preparation Scale (BBPS).
Results
A total of 461 patients were randomized in groups (NaP group: n = 231; PEG group: n = 230). Median age was 54 and 52 in NaP group and PEG group, respectively (p < 0.01). Patients experienced an overall compliance to the treatment in 99.6 and 94.1 % in the NaP group and in the PEG group, respectively (p < 0.001). The mean time of withdrawal was 15.1 ± 8.9 and 15.4 ± 9.5 min in the NaP group and in the PEG group, respectively (p = 0.95). The good quality of bowel preparation, defined as BBPS score ≥7, was obtained in 86.4 and 89.0 % of cases in the NaP group and in the PEG group, respectively (p = 0.42). In all segment (right colon, transverse colon and left colon and rectum), the NaP group was non-inferior to the PEG group. Bowel prep regimen was more frequently considered as “easy” by patients from the NaP group (54.8 % of patients) than patients from the PEG group (29.0 % of patients; p < 0.001). No serious adverse events were reported. No statistical differences were found between the NaP group and the PEG group concerning the incidence of an adverse event (338 vs. 322, respectively).
Conclusion
While NaP tablets appeared as efficient as PEG in terms of colon cleansing prior to a colonoscopy, they significantly improved the overall compliance and eased product administration. At an era where bowel cleansing appears to be the cornerstone of a quality colonoscopy, NaP tablets in patients without contraindication might be considered as an option.

Similar content being viewed by others
References
Coriat R, Lecler A, Lamarque D, Deyra J, Roche H, Nizou C et al (2012) Quality indicators for colonoscopy procedures: a prospective multicentre method for endoscopy units. PLoS One 7(4):e33957
Coriat R, Pommaret E, Chryssostalis A, Viennot S, Gaudric M, Brezault C et al (2009) Quality control of colonoscopy procedures: a prospective validated method for the evaluation of professional practices applicable to all endoscopic units. Gastroenterol Clin Biol 33(2):103–108
Parente F, Marino B, Crosta C (2009) Bowel preparation before colonoscopy in the era of mass screening for colo-rectal cancer: a practical approach. Dig Liver Dis 41(2):87–95
DiPalma JA, Brady CE 3rd, Stewart DL, Karlin DA, McKinney MK, Clement DJ et al (1984) Comparison of colon cleansing methods in preparation for colonoscopy. Gastroenterology 86(5 Pt 1):856–860
Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B et al (2006) A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Gastrointest Endosc 63(7):894–909
Coriat R, Polin V, Oudjit A, Henri F, Dhooge M, Leblanc S et al (2014) Gastric emptying evaluation by ultrasound prior colonoscopy: an easy tool following bowel preparation. World J Gastroenterol 20(37):13591–13598
Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC (2009) The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc 69(3 Pt 2):620–625
Johanson JF, Popp JW Jr, Cohen LB, Lottes SR, Forbes WP, Walker K et al (2007) A randomized, multicenter study comparing the safety and efficacy of sodium phosphate tablets with 2 L polyethylene glycol solution plus bisacodyl tablets for colon cleansing. Am J Gastroenterol 102(10):2238–2246
Rex DK, Schwartz H, Goldstein M, Popp J, Katz S, Barish C et al (2006) Safety and colon-cleansing efficacy of a new residue-free formulation of sodium phosphate tablets. Am J Gastroenterol 101(11):2594–2604
Hagège H, Laugier R, Nahon S, Coulom P, Isnard-Bagnis C, Albert-Marty A (2015) Real-life conditions of use of sodium phosphate tablets for colon cleansing before colonoscopy. Endosc Int Open 3(4):E346–E353
Rex DK (2006) Maximizing detection of adenomas and cancers during colonoscopy. Am J Gastroenterol 101(12):2866–2877
Coriat R, Lecler A, Cassaz C, Roche H, Podevin P, Mesnard B et al (2009) Évaluation des pratiques professionnelles en endoscopie: étude multicentrique de faisabilité d’une méthode simple et reproductible d’évaluation de la qualité de la coloscopie dans des structures privées et publiques. Gastroenterol Clin Biol 33(HS1):175
de Jonge V, Sint Nicolaas J, Cahen DL, Moolenaar W, Ouwendijk RJ, Tang TJ et al (2011) Quality evaluation of colonoscopy reporting and colonoscopy performance in daily clinical practice. Gastrointest Endosc 75(1):98–106
Lee YH, Jeong SY, Kim YS, Jung HJ, Kwon MJ, Kwak CH et al (2015) Randomized controlled trial of sodium phosphate tablets versus 2 L polyethylene glycol solution for bowel cleansing prior colonoscopy. Korean J Gastroenterol 65(1):27–34
Acknowledgments
ICOL121 Investigator Study Group: Dr. Pierre Toulemonde, Clinique Saint-Jean Languedoc, Toulouse, France; Dr. Christoph Schmöcker, Sana Klinikum Lichtenberg, Berlin, Germany; Dr. Pedro Menchén Fernández-Pacheco, Hospital U. Gregorio Marañón, Madrid, Spain; Dr. Miguel Muñoz-Navas, Clínica Universidad Navarra, Pamplona, Spain; Dr. Cédric Lecaille, Polyclinique Bordeaux Nord Aquitaine, Bordeaux, France; Dr. Iván Guerra Marina, Hospital de Fuenlabrada, Madrid, Spain; Dr. Marc Le Rhun, Chu Nantes Hôtel-Dieu, Nantes, France; Dr. Pedro Alonso Aguirre, Complejo Hospitalario A Coruña, A Coruña, Spain; Dr. Stéphane Nahon, Groupe Hospitalier Intercommunal Le Raincy, Montfermeil, France; Pr. Stanislas Chaussade, Hôpital Cochin, Paris, France; Dr. Martin Grünewald, Klinikum Heidenheim Medizinische Klinik I, Heidenheim, Germany; Dr. Geoffroy Vanbiervliet, CHU Nice, Hôpital l’Archet 2, Nice, France; Dr. Carmelo Gómez Gómez, Hospital 12 de Octubre, Madrid, Spain; Pr. Robert Benamouzig, Hôpital Avicenne, Bobigny, France; Dr. Rodica Gincul, Hôpital Edouard Herriot, Lyon, France; Pr. Dr. Franck Thomas Kolligs, Klinikum der Universität München, München, Germany; Dr. María Pellise Urquiza, Hospital Clínic de Barcelona, Barcelona, Spain; Dr. Enrique Rey Díaz-Rubio, Hospital Clínico San Carlos, Madrid, Spain; and Pr. Dr. Von Christian Tirpitz, Kreisklinik Biberach, Biberach, Germany. We extend our gratitude to the patients without whom this study would not have been possible.
Sources of support
The study was supported by Mayoly Splindler.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr V. O’Mahony and Dr. F. Henri are employees of Laboratoires Mayoly Spindler. Stanislas Chaussade, Christoph Schmöcker, Pierre Toulemonde, Miguel Muñoz-Navas, and Valérie O’Mahony have no conflicts of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table 1
Treatment Emergent Adverse Events Experienced by Patients. (DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Chaussade, S., Schmöcker, C., Toulemonde, P. et al. Phosphate tablets or polyethylene glycol for preparation to colonoscopy? A multicentre non-inferiority randomized controlled trial. Surg Endosc 31, 2166–2173 (2017). https://doi.org/10.1007/s00464-016-5214-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5214-1