Abstract
Purpose
The purpose of this study is to compare the efficacy and acceptability of an evening-before regimens of sodium picosulfate/magnesium citrate (SPMC) and polyethylene glycol (PEG) as bowel cleansers and to explore the results of a same-day regimen of SPMC.
Methods
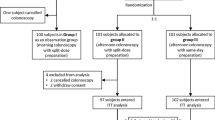
Multicenter, randomized, observer-blinded, parallel study carried out in subjects who were 18–80 years old and were undergoing diagnostic colonoscopy for the first time. The primary outcome was treatment success, which was a composite outcome defined by (1) the evaluation of the overall preparation quality as “excellent” or “good” by two blinded independent evaluators with the Fleet® Grading Scale for Bowel Cleansing and (2) a subject’s acceptability rating of “easy to take” or “tolerable.” The primary outcome was analyzed using a logistic regression with site, gender, and age group (age ≥65 years and <65 years) as factors.
Results
Four hundred ninety subjects were included in the efficacy evaluation. Although treatment success was significantly higher in subjects assigned to the evening-before regimen of SPMC vs. subjects assigned to the evening-before PEG, when evaluating the two individual components for treatment success, there were significant differences in the ease of completion but not in the quality of preparation. The same-day SPMC regimen was superior to both the evening-before regimen of SPMC and PEG in terms of the quality of preparation, especially regarding the proximal colon.
Conclusions
An evening-before regimen of SPMC is superior to an evening-before regimen of PEG in terms of subject’s acceptability. The same-day SPMC regimen provides better cleansing levels in the proximal colon.

Similar content being viewed by others
References
Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM (2009) American college of gastroenterology guidelines for colorectal cancer screening [corrected]. Am J Gastroenterol 104(3):739–750. doi:10.1038/ajg.2009.104
Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B et al (2006) A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Dis Colon Rectum 49(6):792–809
Parente F, Marino B, Crosta C (2009) Bowel preparation before colonoscopy in the era of mass screening for colo-rectal cancer: a practical approach. Dig Liver Dis 41(2):87–95. doi:10.1016/j.dld.2008.06.005
Hoy SM, Scott LJ, Wagstaff AJ (2009) Sodium picosulfate/magnesium citrate: a review of its use as a colorectal cleanser. Drugs 69(1):123–136. doi:10.2165/00003495-200969010-00009
Lawrance IC, Willert RP, Murray K (2011) Bowel cleansing for colonoscopy: prospective randomized assessment of efficacy and of induced mucosal abnormality with three preparation agents. Endoscopy 43(5):412–418. doi:10.1055/s-0030-1256193
Worthington J, Thyssen M, Chapman G, Chapman R, Geraint M (2008) A randomised controlled trial of a new 2 litre polyethylene glycol solution versus sodium picosulphate + magnesium citrate solution for bowel cleansing prior to colonoscopy. Curr Med Res Opin 24(2):481–488. doi:10.1185/030079908X260844
Hamilton D, Mulcahy D, Walsh D, Farrelly C, Tormey WP, Watson G (1996) Sodium picosulphate compared with polyethylene glycol solution for large bowel lavage: a prospective randomised trial. Br J Clin Pract 50(2):73–75
Balaban DH, Leavell BS Jr, Oblinger MJ, Thompson WO, Bolton ND, Pambianco DJ (2003) Low volume bowel preparation for colonoscopy: randomized, endoscopist-blinded trial of liquid sodium phosphate versus tablet sodium phosphate. Am J Gastroenterol 98(4):827–832
Fleiss JL, Cohen J, Everitt BS (1969) Large sample standard errors of kappa and weighed kappa. Psychol Bull 72:323–327
Lawrence EM, Pickhardt PJ (2010) Low-volume hybrid bowel preparation combining saline laxatives with oral contrast agents versus standard polyethylene glycol lavage for colonoscopy. Dis Colon Rectum 53(8):1176–1181. doi:10.1007/DCR.0b013e3181d5d9ac
Church JM (1998) Effectiveness of polyethylene glycol antegrade gut lavage bowel preparation for colonoscopy—timing is the key! Dis Colon Rectum 41(10):1223–1225
Chiu HM, Lin JT, Wang HP, Lee YC, Wu MS (2006) The impact of colon preparation timing on colonoscopic detection of colorectal neoplasms—a prospective endoscopist-blinded randomized trial. Am J Gastroenterol 101(12):2719–2725
Varughese S, Kumar AR, George A, Castro FJ (2010) Morning-only one-gallon polyethylene glycol improves bowel cleansing for afternoon colonoscopies: a randomized endoscopist-blinded prospective study. Am J Gastroenterol 105(11):2368–2374. doi:10.1038/ajg.2010.271
Parra-Blanco A, Nicolas-Perez D, Gimeno-Garcia A, Grosso B, Jimenez A, Ortega J et al (2006) The timing of bowel preparation before colonoscopy determines the quality of cleansing, and is a significant factor contributing to the detection of flat lesions: a randomized study. World J Gastroenterol 12(38):6161–6166
Kilgore TW, Abdinoor AA, Szary NM, Schowengerdt SW, Yust JB, Choudhary A et al (2011) Bowel preparation with split-dose polyethylene glycol before colonoscopy: a meta-analysis of randomized controlled trials. Gastrointest Endosc 73(6):1240–1245. doi:10.1016/j.gie.2011.02.007
Martel M, Barkun AN, Menard C, Restellini S, Kherad O, Vanasse A. (2015) Split-dose preparations are superior to day-before bowel cleansing regimens: a meta-analysis. Gastroenterol. 8 Apr. doi: 10.1053/j.gastro.2015.04.004
Yoo IK, Lee JS, Chun HJ, Jeen YT, Keum B, Kim ES et al (2015) A randomized, prospective trial on efficacy and tolerability of low-volume bowel preparation methods for colonoscopy. Dig Liver Dis 47:131–137
Jeon SR, Kim HG, Lee JS, Kim JO, Lee TH, Cho JH et al (2015) Randomized controlled trial of low-volume bowel preparation agents for colonic bowel preparation: 2L polyethylene glycol with ascorbic acid vs sodium picosulfate with magnesium citrate. Int J Color Dis 30:251–258
Siddiqui AA, Yang K, Spechler SJ, Cryer B, Davila R, Cipher D et al (2009) Duration of the interval between the completion of bowel preparation and the start of colonoscopy predicts bowel-preparation quality. Gastrointest Endosc 69(3 Pt 2):700–706. doi:10.1016/j.gie.2008.09.047
Brady M, Kinn S, Stuart P. (2003) Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev. (4) (4):CD004423.
Dalal KS, Rajwade D, Suchak R (2010) “Nil per oral after midnight”: is it necessary for clear fluids? Indian J Anaesthesiol 54(5):445–447
Huffman M, Unger RZ, Thatikonda C, Amstutz S, Rex DK (2010) Split-dose bowel preparation for colonoscopy and residual gastric fluid volume: an observational study. Gastrointest Endosc 72:516–522
American Society of Anesthesiologists Committee (2011) Practice Guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology 114:495–511
Smith I, Kranke P, Murat I, Smith A, O’Sullivan G, Soreide E et al (2011) Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol 28:556–569
Prieto-Frías C, Angós R, Betés MT, De la Riva S, Rodríguez-Lago I, Carretero C et al (2014) Split-dose sodium picosulfate and magnesium citrate preparation for colonoscopy: cleansing effectiveness and residual gastric volume and pH. Gastrointest Endosc 79:AB318
Lasisi F, Rex DK (2011) Improving protection against proximal colon cancer by colonoscopy. Expert Rev Gastroenterol Hepatol 5(6):745–754. doi:10.1586/egh.11.78
Wong R (2010) Proximal tumors are associated with greater mortality in colon cancer. J Gen Intern Med 25(11):1157–1163. doi:10.1007/s11606-010-1460-4
Gupta T, Mandot A, Desai D, Abraham P, Joshi A, Shah S (2007) Comparison of two schedules (previous evening versus same morning) of bowel preparation for colonoscopy. Endoscopy 39(8):706–709
Aoun E, Abdul-Baki H, Azar C, Mourad F, Barada K, Berro Z et al (2005) A randomized single-blind trial of split-dose PEG-electrolyte solution without dietary restriction compared with whole dose PEG-electrolyte solution with dietary restriction for colonoscopy preparation. Gastrointest Endosc 62(2):213–218
Park JS, Sohn CI, Hwang SJ, Choi HS, Park JH, Kim HJ et al (2007) Quality and effect of single dose versus split dose of polyethylene glycol bowel preparation for early-morning colonoscopy. Endoscopy 39(7):616–619
Flemming JA, Vanner SJ, Hookey LC (2012) Split-dose picosulfate, magnesium oxide, and citric acid solution markedly enhances colon cleansing before colonoscopy: a randomized, controlled trial. Gastrointest Endosc 75(3):537–544. doi:10.1016/j.gie.2011.09.018
Martin-Noguerol EM, González-Santiago JM, Martínez-Alcalá C, Vinagre-Rodríguez J, Hernández-Alonso M, Dueñas-Sadornil C et al (2013) Split dose sodium picosulfate/magnesium citrate for morning colonoscopies performed 2 to 6 hours after fluid intake. Gastroenterol Hepatol 36:254–260
Manes G, Repici A, Hassan C, MAGIC-P study group (2014) Randomized controlled trial comparing efficacy and acceptability of split- and standard-dose sodium picosulfate plus magnesium citrate for bowel cleansing prior to colonoscopy. Endoscopy 46:662–669
Connor A, Tolan D, Hughes S, Carr N, Tomson C (2012) Consensus guidelines for the safe prescription and administration of oral bowel-cleansing agents. Gut 61(11):1525–1532
Longcroft-Wheaton G, Bhandari P (2012) Same-day bowel cleansing regimen is superior to a split-dose regimen over 2 days for afternoon colonoscopy: results from a large prospective series. J Clin Gastroenterol 46(1):57–61. doi:10.1097/MCG.0b013e318233a986
Cho YS, Nam KM, Park JH, Byun SH, Ryu JS, Kim HJ (2014) Acute hyponatremia with seizure and mental change after oral sodium picosulfate/magnesium citrate bowel preparation. Ann Coloproctol 30:290–293
Levey JM (2012) Same day prep for afternoon colonoscopy: everybody wins! J Clin Gastroenterol 46(1):4–5. doi:10.1097/MCG.0b013e31823a4b44
Acknowledgments
The authors would like to thank Dr. Fernando Rico-Villademoros (APICES, Madrid, Spain) for the support given in Medical Writing of the manuscript, Ángel Callejo (APICES, Madrid, Spain) for coordinating all tasks performed by the different parties during the study conduction, and Dr. José Luis Lledó and Dr. Guillermo Cacho (Madrid, Spain) for the independent blinded evaluations.
The authors also thank to the investigators who participated in this study: Federico Argüelles-Arias (Sevilla, Spain), Lidia Argüello (Valencia, Spain), Raquel Barranco (Valdemoro, Spain), Susana Basterra (Bilbao, Spain), Mª Rosa Briz (Valdemoro, Spain), Ángel José Calderón (Bilbao, Spain), Almudena Calvache (Valdemoro, Spain), Henry R. Cordova (Barcelona, Spain), Joan Dot (Barcelona, Spain), Rosario Fernández (Oviedo, Spain), Raquel García Arcones (Majadahonda, Spain), Blas José Gómez-Rodríguez (Sevilla, Spain), Cecilia González (Madrid, Spain), Ángel González (Córdoba, Spain), Lander Hijona (Bilbao, Spain), Josep Llach (Barcelona, Spain), Oscar Nogales (Madrid, Spain), Akiko Ono (Murcia, Spain), María Antonia Palacio (Oviedo, Spain), Virginia Pertejo (Valencia, Spain), Marta Ponce (Valencia, Spain), Cesar Prieto de Frías (Pamplona, Spain), Enrique Quintero (La Laguna, Spain), María Rodríguez (Oviedo, Spain), Juan Ruíz (Málaga, Spain), Teresa Sala (Valencia, Spain), Luis Vázquez (Málaga, Spain), and Paz Zaballa (Oviedo, Spain).
Compliance with ethical standards
ᅟ
Funding
This study was fully funded by Laboratorios Casen-Fleet S.L.U. (currently Casen Recordati S.L.)
Conflict of interest
The authors declare that they have no competing interests.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muñoz-Navas, M., Calleja, J.L., Payeras, G. et al. A randomized trial to compare the efficacy and tolerability of sodium picosulfate-magnesium citrate solution vs. 4 L polyethylene glycol solution as a bowel preparation for colonoscopy. Int J Colorectal Dis 30, 1407–1416 (2015). https://doi.org/10.1007/s00384-015-2307-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-015-2307-6