Abstract
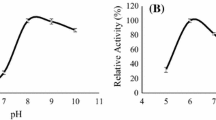
The use of chicken waste can contribute to the development of new processes and obtaining molecules with high added value. An experimental design was applied to evaluate the effect of moisture, temperature, and inoculum size on the production of antioxidant peptides and proteases by A. oryzae IOC3999 through solid-state fermentation (SSF) of chicken viscera meal. As a result, the process conditions strongly influenced protease production and antioxidant activity of the fermented products. A global analysis of the results indicated that the most adequate conditions for SSF were (assay 9): 40% initial moisture, 30 °C as the incubation temperature, 5.05 × 106 spores/g as the inoculum size, and 48-h fermentation as the fermentation time. Under this condition, the antioxidant activities for the ABTS- and DPPH-radicals inhibition and ferric reducing antioxidant power (FRAP) methods were 376.16, 153.29, and 300.47 (µmol TE/g), respectively, and the protease production reached 428.22 U/g. Ultrafiltration of the crude extract obtained under optimized fermentation conditions was performed, and the fraction containing peptides with molecular mass lower than 3 kDa showed the highest antioxidant activity. The proteases were biochemically characterized and showed maximal activity at pH values ranging from 5.0 to 6.0 and a temperature of 50 °C. The thermodynamic parameters indicated that the process of thermal protease inactivation is not spontaneous (ΔG*d > 88.78 kJ/mol), increasing with temperature (ΔH*d 27.01–26.88 kJ/mol), and with reduced disorder in the system (ΔS*d < − 197.74 kJ/mol) probably caused by agglomeration of partially denatured enzymes.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Thoresen PP, Álvarez RG, Vaka MR et al (2020) Potential of innovative pre-treatment technologies for the revalorisation of residual materials from the chicken industry through enzymatic hydrolysis. Innov Food Sci Emerg Technol 64:102377. https://doi.org/10.1016/j.ifset.2020.102377
Ware A, Power N (2017) Modelling methane production kinetics of complex poultry slaughterhouse wastes using sigmoidal growth functions. Renew Energy 104:50–59. https://doi.org/10.1016/j.renene.2016.11.045
Singh P, Mondal T, Sharma R et al (2018) Poultry waste management. Poult Waste Manag 7:694–700. https://doi.org/10.20546/ijcmas.2018.708.077
Ferreira A, Kunh SS, Cremonez PA et al (2018) Brazilian poultry activity waste: destinations and energetic potential. Renew Sustain Energy Rev 81:3081–3089. https://doi.org/10.1016/j.rser.2017.08.078
Alofa CS, Abou Y (2020) A mixture of chicken viscera, housefly larvae and spirulina waste as a mixture of chicken viscera, housefly larvae and spirulina waste as replacement of fishmeal in Nile Tilapia (Oreochromis niloticus) diets. Aquac Stud 21(1):10–21. https://doi.org/10.4194/2618-6381-v21
da Silva VG, de Castro RJS (2018) Biocatalytic action of proteases in ionic liquids: improvements on their enzymatic activity, thermal stability and kinetic parameters. Int J Biol Macromol 114:124–129. https://doi.org/10.1016/j.ijbiomac.2018.03.084
Sadh PK, Duhan S, Duhan JS (2018) Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour Bioprocess 5:1–15. https://doi.org/10.1186/s40643-017-0187-z
Leite P, Belo I, Salgado JM (2021) Co-management of agro-industrial wastes by solid-state fermentation for the production of bioactive compounds. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2021.113990
Verduzco-Oliva R, Gutierrez-Uribe JA (2020) Beyond enzyme production: solid state fermentation (SSF) as an alternative approach to produce antioxidant polysaccharides. Sustain. https://doi.org/10.3390/su12020495
Chilakamarry CR, Mimi Sakinah AM, Zularisam AW et al (2022) Advances in solid-state fermentation for bioconversion of agricultural wastes to value-added products: opportunities and challenges. Bioresour Technol 343:126065. https://doi.org/10.1016/j.biortech.2021.126065
Hubka V, Kolařík M, Kubátová A, Peterson SW (2013) Taxonomic revision of eurotium and transfer of species to aspergillus. Mycologia 105:912–937. https://doi.org/10.3852/12-151
Park HS, Jun SC, Han KH et al (2017) Diversity, application, and synthetic biology of industrially important Aspergillus Fungi. Adv Appl Microbiol 100:161–202. https://doi.org/10.1016/bs.aambs.2017.03.001
Kumar V, Ahluwalia V, Saran S et al (2021) Recent developments on solid-state fermentation for production of microbial secondary metabolites: challenges and solutions. Bioresour Technol 323:124566. https://doi.org/10.1016/j.biortech.2020.124566
Ohara A, Gonçalves J, Alencar J et al (2018) A multicomponent system based on a blend of agroindustrial wastes for the simultaneous production of industrially applicable enzymes by solid-state fermentation. Food Sci Technol 2061:131–137. https://doi.org/10.1590/1678-457X.17017
Melnichuk N, Braia MJ, Anselmi PA et al (2020) Valorization of two agroindustrial wastes to produce alpha-amylase enzyme from Aspergillus oryzae by solid-state fermentation. Waste Manag 106:155–161. https://doi.org/10.1016/j.wasman.2020.03.025
Dias LM, dos Santos BV, Albuquerque CJB et al (2017) Biomass sorghum as a novel substrate in solid-state fermentation for the production of hemicellulases and cellulases by Aspergillus niger and A. fumigatus. J Appl Microbiol 124:708–718
Jiménez-Quero A, Pollet E, Avérous L, Phalip V (2020) Optimized bioproduction of itaconic and fumaric acids based on solid-state fermentation of lignocellulosic biomass. Molecules. https://doi.org/10.3390/molecules25051070
Dessie W, Zhang W, Xin F et al (2018) Succinic acid production from fruit and vegetable wastes hydrolyzed by on-site enzyme mixtures through solid state fermentation. Bioresour Technol 247:1177–1180. https://doi.org/10.1016/j.biortech.2017.08.171
Chen X, Yan J, Chen J et al (2021) Potato pomace: an efficient resource for Monascus pigments production through solid-state fermentation. J Biosci Bioeng 132:167–173. https://doi.org/10.1016/j.jbiosc.2021.03.007
Martínez O, Sánchez A, Font X, Barrena R (2018) Bioproduction of 2-phenylethanol and 2-phenethyl acetate by Kluyveromyces marxianus through the solid-state fermentation of sugarcane bagasse. Appl Microbiol Biotechnol 102:4703–4716. https://doi.org/10.1007/s00253-018-8964-y
Jiang X, Cui Z, Wang L et al (2020) Production of bioactive peptides from corn gluten meal by solid-state fermentation with Bacillus subtilis MTCC5480 and evaluation of its antioxidant capacity in vivo. Lwt 131:109767. https://doi.org/10.1016/j.lwt.2020.109767
da Silva OS, de Oliveira RL, Souza-Motta CM et al (2016) Novel protease from Aspergillus tamarii URM4634: production and characterization using inexpensive agroindustrial substrates by solid-state fermentation. Adv Enzym Res 04:125–143. https://doi.org/10.4236/aer.2016.44012
Nasri M (2017) Protein hydrolysates and biopeptides: production, biological activities, and applications in foods and health benefits: a review, 1st edn. Elsevier, London
Chai KF, Voo AYH, Chen WN (2020) Bioactive peptides from food fermentation: a comprehensive review of their sources, bioactivities, applications, and future development. Compr Rev Food Sci Food Saf 19:3825–3885. https://doi.org/10.1111/1541-4337.12651
Clerici NJ, Lermen AM, Daroit DJ (2021) Agro-industrial by-products as substrates for the production of bacterial protease and antioxidant hydrolysates. Biocatal Agric Biotechnol. https://doi.org/10.1016/j.bcab.2021.102174
Tuly JA, Zabed HM, Nizami AS et al (2022) Bioconversion of agro-food industrial wastes into value-added peptides by a Bacillus sp. Mutant through solid-state fermentation. Bioresour Technol 346:126513. https://doi.org/10.1016/j.biortech.2021.126513
Bin ZT, He TP, Bin LH et al (2016) The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 21:1–14. https://doi.org/10.3390/molecules21010072
Charney J, Tomarelli RM (1947) A colorimetric method for the determination of the proteolytic activity of duodenal juice. J Biol Chem 171:501–505. https://doi.org/10.1016/s0021-9258(17)41059-3
De Castro RJS, Sato HH (2013) Synergistic effects of agroindustrial wastes on simultaneous production of protease and α-amylase under solid state fermentation using a simplex centroid mixture design. Ind Crops Prod 49:813–821. https://doi.org/10.1016/j.indcrop.2013.07.002
De Castro RJS, Sato HH (2014) Production and biochemical characterization of protease from Aspergillus oryzae: An evaluation of the physical-chemical parameters using agroindustrial wastes as supports. Biocatal Agric Biotechnol 3:20–25. https://doi.org/10.1016/j.bcab.2013.12.002
Al-Duais M, Müller L, Böhm V, Jetschke G (2009) Antioxidant capacity and total phenolics of Cyphostemma digitatum before and after processing: use of different assays. Eur Food Res Technol 228:813–821. https://doi.org/10.1007/s00217-008-0994-8
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of ‘“Antioxidant Power ”’: the FRAP assay. Anal Biochem 76:70–76
de Matos FM, Novelli PK, de Castro RJS (2021) Enzymatic hydrolysis of black cricket (Gryllus assimilis) proteins positively affects their antioxidant properties. J Food Sci 86:571–578. https://doi.org/10.1111/1750-3841.15576
de Castro RJS, Ohara A, Nishide TG et al (2015) A new approach for proteases production by Aspergillus niger based on the kinetic and thermodynamic parameters of the enzymes obtained. Biocatal Agric Biotechnol 4:199–207. https://doi.org/10.1016/j.bcab.2014.12.001
Amaral YMS, da Silva OS, de Oliveira RL, Porto TS (2020) Production, extraction, and thermodynamics protease partitioning from Aspergillus tamarii Kita UCP1279 using PEG/sodium citrate aqueous two-phase systems. Prep Biochem Biotechnol 50:619–626. https://doi.org/10.1080/10826068.2020.1721535
Benabda O, Sana M, Kasmi M et al (2019) Optimization of protease and amylase production by Rhizopus oryzae cultivated on bread waste using solid-state fermentation. J Chem 2019:1–9
Balachandran C, Vishali A, Arun N, Baskar K (2021) Optimization of protease production from Bacillus halodurans under solid state fermentation using agrowastes. Saudi J Biol Sci 28:4263–4269. https://doi.org/10.1016/j.sjbs.2021.04.069
Yoon LW, Ang TN, Ngoh GC, Chua ASM (2014) Fungal solid-state fermentation and various methods of enhancement in cellulase production. Biomass Bioenerg 67:319–338. https://doi.org/10.1016/j.biombioe.2014.05.013
Magro AEA, Silva LC, Rasera GB, de Castro RJS (2019) Solid-state fermentation as an efficient strategy for the biotransformation of lentils: enhancing their antioxidant and antidiabetic potentials. Bioresour Bioprocess. https://doi.org/10.1186/s40643-019-0273-5
Leite P, Silva C, Salgado JM, Belo I (2019) Simultaneous production of lignocellulolytic enzymes and extraction of antioxidant compounds by solid-state fermentation of agro-industrial wastes. Ind Crops Prod 137:315–322. https://doi.org/10.1016/j.indcrop.2019.04.044
Punia S, Sandhu KS, Grasso S et al (2021) Aspergillus oryzae fermented rice bran: a byproduct with enhanced bioactive compounds and antioxidant potential. Foods. https://doi.org/10.3390/foods10010070
Yin Z, Wu W, Sun C et al (2018) Comparison of releasing bound phenolic acids from wheat bran by fermentation of three Aspergillus species. Int J Food Sci Technol 53:1120–1130. https://doi.org/10.1111/ijfs.13675
Kumari R, Sharma N, Sharma S et al (2023) Production and characterization of bioactive peptides in fermented soybean meal produced using proteolytic Bacillus species isolated from kinema. Food Chem 421:136130. https://doi.org/10.1016/j.foodchem.2023.136130
Liu F, Chen Z, Shao J et al (2017) Effect of fermentation on the peptide content, phenolics and antioxidant activity of defatted wheat germ. Food Biosci 20:141–148. https://doi.org/10.1016/j.fbio.2017.10.002
Wen C, Zhang J, Zhang H et al (2020) Plant protein-derived antioxidant peptides: Isolation, identification, mechanism of action and application in food systems: a review. Trends Food Sci Technol 105:308–322. https://doi.org/10.1016/j.tifs.2020.09.019
Ketnawa S, Wickramathilaka M, Liceaga AM (2018) Changes on antioxidant activity of microwave-treated protein hydrolysates after simulated gastrointestinal digestion: purification and identification. Food Chem 254:36–46. https://doi.org/10.1016/j.foodchem.2018.01.133
Najafian L, Babji AS (2018) Fractionation and identification of novel antioxidant peptides from fermented fish (Pekasam). J Food Meas Charact 12:2174–2183. https://doi.org/10.1007/s11694-018-9833-1
Panchal G, Hati S, Sakure A (2020) Characterization and production of novel antioxidative peptides derived from fermented goat milk by L. fermentum. LWT 119:108887. https://doi.org/10.1016/j.lwt.2019.108887
Thapa S, Li H, OHair J et al (2019) Biochemical characteristics of microbial enzymes and their significance from industrial perspectives. Mol Biotechnol 61:579–601. https://doi.org/10.1007/s12033-019-00187-1
Da Silva AC, Da Silva EFT, de Queiroz AESF et al (2021) Production, biochemical characterization, and kinetic/thermodynamic study of novel serine protease from Aspergillus avenaceus URM 6706. Biotechnol Prog 37:e3091. https://doi.org/10.1002/btpr.3091
Fernandes LMG, Carneiro-da-Cunha MN, de Silva JC et al (2020) Purification and characterization of a novel Aspergillus heteromorphus URM 0269 protease extracted by aqueous two-phase systems PEG/citrate. J Mol Liq 317:113957. https://doi.org/10.1016/j.molliq.2020.113957
Kaewsalud T, Yakul K, Jantanasakulwong K et al (2021) Biochemical characterization and application of thermostable-alkaline keratinase from Bacillus halodurans SW-X to Valorize Chicken Feather Wastes. Waste and Biomass Valorization 12:3951–3964. https://doi.org/10.1007/s12649-020-01287-9
Coban HB (2022) Modification of Aspergillus niger ATCC11414 growth for the enhancement of protease production by the effect of natural microparticles. Middle East J Sci 8:99–105. https://doi.org/10.51477/mejs.1109174
De ORL, Claudino EDS, Converti A (2021) Use of a sequential fermentation method for the production of Aspergillus tamarii URM4634 protease and a kinetic/thermodynamic study of the enzyme. Catalysts 11:1–15
de Oliveira RL, dos Santos VLV, da Silva MF, Porto TS (2020) Kinetic/thermodynamic study of immobilized β-fructofuranosidase from Aspergillus tamarii URM4634 in chitosan beads and application on invert sugar production in packed bed reactor. Food Res Int 137:109730. https://doi.org/10.1016/j.foodres.2020.109730
dos Santos Aguilar JG, de Souza AKS, de Castro RJS (2020) Enzymatic hydrolysis of chicken viscera to obtain added-value protein hydrolysates with antioxidant and antihypertensive properties. Int J Pept Res Ther 26:717–725. https://doi.org/10.1007/s10989-019-09879-3
Soares da Silva O, Lira de Oliveira R, de Carvalho SJ et al (2018) Thermodynamic investigation of an alkaline protease from Aspergillus tamarii URM4634: a comparative approach between crude extract and purified enzyme. Int J Biol Macromol 109:1039–1044. https://doi.org/10.1016/j.ijbiomac.2017.11.081
de Oliveira RL, da Silva MF, Converti A, Porto TS (2019) Biochemical characterization and kinetic/thermodynamic study of Aspergillus tamarii URM4634 β-fructofuranosidase with transfructosylating activity. Biotechnol Prog 35:1–12. https://doi.org/10.1002/btpr.2879
de Silva JC, de França PRL, Converti A, Porto TS (2018) Kinetic and thermodynamic characterization of a novel Aspergillus aculeatus URM4953 polygalacturonase. Comparison of free and calcium alginate-immobilized enzyme. Process Biochem 74:61–70. https://doi.org/10.1016/j.procbio.2018.07.010
Chauhan JV, Mathukiya RP, Singh SP, Gohel SD (2021) Two steps purification, biochemical characterization, thermodynamics and structure elucidation of thermostable alkaline serine protease from Nocardiopsis alba strain OM-5. Int J Biol Macromol 169:39–50. https://doi.org/10.1016/j.ijbiomac.2020.12.061
de Oliveira RL, da Silva MF, da Silva SP et al (2020) Immobilization of a commercial Aspergillus aculeatus enzyme preparation with fructosyltransferase activity in chitosan beads: a kinetic/thermodynamic study and fructo-oligosaccharides continuous production in enzymatic reactor. Food Bioprod Process 122:169–182. https://doi.org/10.1016/j.fbp.2020.05.001
Singh AK, Chhatpar HS (2011) Purification, characterization and thermodynamics of antifungal protease from Streptomyces sp. A6. J Basic Microbiol 51:424–432. https://doi.org/10.1002/jobm.201000310
Souza PM, Aliakbarian B, Filho EXF et al (2015) Kinetic and thermodynamic studies of a novel acid protease from Aspergillus foetidus. Int J Biol Macromol 81:17–21. https://doi.org/10.1016/j.ijbiomac.2015.07.043
Avwioroko OJ, Anigboro AA, Ejoh AS et al (2019) Characterization of α-amylases isolated from Cyperus esculentus seeds (tigernut): biochemical features, kinetics and thermal inactivation thermodynamics. Biocatal Agric Biotechnol 21:101298. https://doi.org/10.1016/j.bcab.2019.101298
Hernández-Martínez R, Gutiérrez-Sánchez G, Bergmann CW et al (2011) Purification and characterization of a thermodynamic stable serine protease from Aspergillus fumigatus. Process Biochem 46:2001–2006. https://doi.org/10.1016/j.procbio.2011.07.013
Abdel-Naby MA, Ahmed SA, Wehaidy HR, El-Mahdy SA (2017) Catalytic, kinetic and thermodynamic properties of stabilized Bacillus stearothermophilus alkaline protease. Int J Biol Macromol 96:265–271. https://doi.org/10.1016/j.ijbiomac.2016.11.094
Acknowledgements
This study was financed by the Coordination of Improvement of Higher Education Personnel—Brazil (CAPES) (Finance Code 001). Acknowledgments to Ad'oro Company for gently donating chicken viscera flour.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amaral, Y.M.S., de Castro, R.J.S. Chicken viscera meal as substrate for the simultaneous production of antioxidant compounds and proteases by Aspergillus oryzae. Bioprocess Biosyst Eng 46, 1777–1790 (2023). https://doi.org/10.1007/s00449-023-02934-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02934-w