Abstract
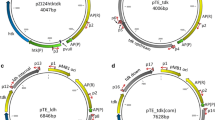
Gene-expression cassettes for the construction of recombinant Clostridium beijerinckii were developed as potential tools for metabolic engineering of C. beijerinckii. Gene expression cassettes containing ColE1 origin and pAMB origin along with the erythromycin resistance gene were constructed, in which promoters from Escherichia coli, Lactococcus lactis, Ralstonia eutropha, C. acetobutylicum, and C. beijerinckii are examined as potential promoters in C. beijerinckii. Zymogram analysis of the cell extracts and comparison of lipase activities of the recombinant C. beijerinckii strains expressing Pseudomonas fluorescens tliA gene suggested that the tliA gene was functionally expressed by all the examined promoters with different expression level. Also, recombinant C. beijerinckii expressing C. beijerinckii secondary alcohol dehydrogenase by the constructed expression cassettes successfully produced 2-propanol from glucose. The best promoter for TliA expression was the R. eutropha phaP promoter while that for 2-propanol production was the putative C. beijerinckii pta promoter. Gene expression cassettes developed in this study may be useful tools for the construction of recombinant C. beijerinckii strains as host strains for the valuable chemicals and fuels from renewable resources.




Similar content being viewed by others
References
Cornillot E, Nair RV, Papoutsakis ET, Soucaille P (1995) The genes for butanol and acetone formation in Clostridium acetobutylicum ATCC 824 reside on a large plasmid whose loss leads to degeneration of the strain. J Bacteriol 179:5442–5447
Formanek J, Mackie R, Blaschek HP (1997) Enhanced butanol production by Clostridium beijerinckii BA101 grown in semidefined P2 medium containing 6 % maltodextrin or glucose. Appl Environ Microbiol 63:2306–2310
Keis S, Shaheen R, Jones DT (2001) Emended descriptions of Clostridium acetobutylicum and Clostridium beijerinckii, and descriptions of Clostridium saccharoperbutylacetonicum sp. nov. and Clostridium saccharobutylicum sp. nov. Int J Syst Evol Microbiol 51:2095–2103
Reid SJ, Rafudeen SM, Rafudeen S, Leat NG (1999) The genes controlling sucrose utilization in CIostridiunr beuerinckii NCIMB 8052 constitute an operon. Microbiology 145:1461–1472
Wilkinson SR, Young M (1995) Physical map of the Clostridium beijerinckii (formerly Clostridium acetobutylicum) NCIMB 8052 chromosome. J Bacteriol 177:439–448
Borden JR, Papoutsakis ET (2007) Dynamics of genomic-library enrichment and identification of solvent tolerance genes for Clostridium acetobutylicum. Appl Environ Microbiol 73:3061–3068
Green EM (2011) Fermentative production of butanol-the industrial perspective. Curr Opin Biotechnol 22:337–343
Harris LM, Desai RP, Welker NE, Papoutsakis ET (2000) Characterization of recombinant strains of the Clostridium acetobutylicum butyrate kinase inactivation mutant: need for new phenomenological models for solventogenesis and butanol inhibition? Biotechnol Bioeng 67:1–11
Harris LM, Blank L, Desai RP, Welker NE, Papoutsakis ET (2001) Fermentation characterization and flux analysis of recombinant strains of Clostridium acetobutylicum with an inactivated solR gene. J Ind Microbiol Biotechnol 27:322–328
Mingardon F, Chanal A, Tardif C, Fierobe HP (2011) The issue of secretion in heterologous expression of Clostridium cellulolyticum cellulase-encoding genes in Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 77:2831–2838
Feustel L, Nakotte S, Dürre P (2004) Characterization and development of two reporter gene systems for Clostridium acetobutylicum. Appl Environ Microbiol 70:798–803
Girbal L, Mortier-Barrière I, Raynaud F, Rouanet C, Croux C, Soucaille P (2003) Development of a sensitive gene expression reporter system and an inducible promoter-repressor system for Clostridium acetobutylicum. Appl Environ Microbiol 69:4985–4988
Nair RV, Papoutsakus ET (1994) Expression of plasmid-encoded aad in Clostridium acetobutylicum M5 restores vigorous butanol production. J Bacteriol 176:5843–5846
Scotcher MC, Bennett GN (2008) Activity of abrB310 promoter in wild type and spo0A-deficient strains of Clostridium acetobutylicum. J Ind Microbiol Biotechnol 35:743–750
Wang Y, Li X, Milne CB, Janssen H, Lin W, Phan G, Hu H, Jin YS, Price ND, Blaschek HP (2013) Development of a gene knockout system using mobile group II introns (Targetron) and genetic disruption of acid production pathways in Clostridium beijerinckii. Appl Environ Microbiol 79:5853–5863
Wang Y, Zhang ZT, Seo SO, Choi K, Lu T, Jin YS, Blaschek HP (2015) Markerless chromosomal gene deletion in Clostridium beijerinckii using CRISPR/Cas9 system. J Biotechnol 20:1–5
Li GS (1998) Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA. Development of a reporter system for the study of gene expression for solvent production in Clostridium beijerinckii NRRL B592 and Clostridium acetobutylicum ATCC 824
López-Contreras AM, Smidt H, van der Oost J, Claassen PA, Mooibroek H, de Vos WM (2001) Clostridium beijerinckii cells expressing Neocallimastix patriciarum glycoside hydrolases show enhanced lichenan utilization and solvent production. Appl Environ Microbiol 67:5127–5133
Quixley KW, Reid SJ (2000) Construction of a reporter gene vector for Clostridium beijerinckii using a Clostridium endoglucanase gene. J Mol Microbiol Biotechnol 2:53–57
Xiao H, Li Z, Jiang Y, Yang Y, Jiang W, Gu Y, Yang S (2012) Metabolic engineering of D-xylose pathway in Clostridium beijerinckii to optimize solvent production from xylose mother liquid. Metab Eng 14:569–578
Israelsen H, Madsen SM, Vrang A, Hansen EB, Johansen E (1995) Cloning and partial characterization of regulated promoters from Lactococcus lactis Tn917-lacZ integrants with the new promoter probe vector pAK80. Appl Environ Microbiol 61:2540–2547
Peekhaus N, Conway T (1998) Positive and negative transcriptional regulation of the Escherichia coli gluconate regulon gene gntT by GntR and the cyclic AMP (cAMP)-cAMP receptor protein complex. J Bacteriol 180:1777–1785
van der Vossen JM, Lelie D, Venema G (1987) Isolation and characterization of Streptococcuts cremnoris Wg2-specific promoters. Appl Environ Microbiol 53:2452–2457
York GM, Stubbe J, Sinskey AJ (2001) New insight into the role of the PhaP phasin of Ralstonia eutropha in promoting the synthesis of polyhydroxybutyrate. J Bacteriol 183:2394–2397
Park SJ, Lee TW, Lim SC, Kim TW, Lee H, Kim MK, Lee SH, Song BK, Lee SY (2012) Biosynthesis of polyhydroxyalkanoates containing 2-hydroxybutyrate from unrelated carbon source by metabolically engineered Escherichia coli. Appl Microbiol Biotechnol 93:273–283
Oultram JD, Loughlin M, Swinfield TJ, Brehm JK, Thompson DE, Minton NP (1988) Introduction of plasmids into whole cells of Clostridium acetobutylicum by electroporation. FEMS Microbiol Lett 56:83–88
Oh YH, Eom GT, Kang KH, Choi JW, Song BK, Lee SH, Park SJ (2015) Optimized transformation of newly constructed Escherichia coli-Clostridia shuttle vectors into Clostridium beijerinckii. Appl Biochem Biotechnol 177:226–236
Chung GH, Lee YP, Jeohn GH, Yoo OJ, Rhee JS (1991) Cloning and Nucleotide sequence of thermostable lipase gene from Pseudomonas fluorescens SIK W1. Agric Boil Chem 55:2359–2365
Edwards AN, Pascual RA, Childress KO, Nawrocki KL, Woods EC, McBride SM (2015) An alkaline phosphatase reporter for use in Clostridium difficile. Anaerobe 32:98–104
Ezeji TC, Qureshi N, Blaschek HP (2007) Bioproduction of butanol from biomass: from genes to bioreactors. Curr Opin Biotechnol 18:220–227
Park SJ, Park JP, Lee SY (2002) Metabolic engineering of Escherichia coli for the production of medium-chain-length polyhydroxyalkanoates rich in specific monomers. FEMS Microbiol Lett 214:217–222
Park SJ, Lee SY (2003) Identification and characterization of a new enoyl-coA hydratase involved in the biosynthesis of medium-chain-length polyhydroxyalkanoates in recombinant Escherichia coli. J Bacteriol 185:5391–5397
Park SJ, Lee SH, Oh YH, Lee SY (2015) Establishment of biosynthesis pathway for (R)-3-hydroxyalkanoates in recombinant Escherichia coli. Korean J Chem Eng 32:702–706
D’Souza R, Pandeya DR, Hong ST (2012) Review: Lactococcus lactis: an efficient gram positive cell factory for the production and secretion of recombinant protein. Biomed Res 23:1–7
Siemerink MAJ, Kuit W, Contreras AML, Eggink G, van der Oost J, Kengen SW (2011) d-2,3-Butanediol production due to heterologous expression of an acetoin reductase in Clostridium acetobutylicum. Appl Environ Microbiol 77:2582–2588
Mingardon F, Perret S, Bélaïch A, Tardif C, Bélaïch J, Fierobe H (2005) Heterologous production, assembly, and secretion of a minicellulosome by Clostridium acetobytylicum ATCC 824. Appl Environ Microbiol 71:1215–1222
Oh YH, Eom IY, Joo JC, Yu JH, Song BK, Lee SH, Hong SH, Park SJ (2015) Recent advances in development of biomass pretreatment technologies used in biorefinery for the production of bio-based fuels, chemicals and polymers. Korean J Chem Eng 32:1945–1959
Acknowledgments
This work was supported by the R&D Program of MOTIE/KEIT (10049674) and 2014 Research Fund of Myongji University.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Oh, Y.H., Eom, G.T., Kang, K.H. et al. Construction of heterologous gene expression cassettes for the development of recombinant Clostridium beijerinckii . Bioprocess Biosyst Eng 39, 555–563 (2016). https://doi.org/10.1007/s00449-016-1537-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1537-5