Abstract
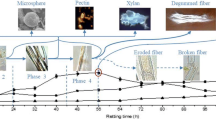
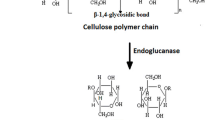
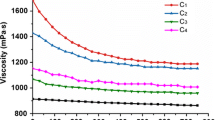
Degummed ramie fiber is widely used in the textile industry. Cellulase enzyme can be effectively used for bio-polishing of the ramie fiber. We immobilized Agrobacterium larrymoorei A1, a potent extra-cellular cellulase producing bacteria, in Ca-alginate. The production of enzyme significantly increased with increasing alginate concentration and reached a maximum activity of 0.28 IU/ml at 20 g/l, which was 32 % higher as compared to free cells. These immobilized cells were used on ramie fibers. Scanning electron micrograph (SEM) and differential interference contrast (DIC) studies showed increased smoothness and orientation of surface structure of the fibers after 19.5 h. The single fiber tenacity was almost same as compared to non-treated fiber and the initial modulus increased by 24.01 %. The remarkable reusability of these immobilized cells provides a cost effective method for treatment of natural fibers containing cellulose.








Similar content being viewed by others
References
Begum F, Absar N, Alam MS (2009) Purification and characterization of extracellular cellulase from A. oryzae ITCC-4857.01. J Appl Sci Res 5:1645–1651
Dienes D, Egyhazi A, Reczey K (2004) Treatment of recycled fiber with trichoderma cellulases. Ind Crop Prod 20(1):11–21. doi:10.1016/j.indcrop.2003.12.009
Kaur B, Oberoi HS, Chadha BS (2014) Enhanced cellulase producing mutants developed from heterokaryotic Aspergillus strain. Bioresour Technol 156C:100–107. doi:10.1016/j.biortech.2014.01.016
Sukumaran RK, Singhania RR, Pandey A (2005) Microbial cellulases—production, applications and challenges. J Sci Ind Res 64:832–844
Wang CY, Hsieh YR, Ng CC, Chan H, Lin HT, Tzeng WS, Shyu YT (2009) Purification and characterization of a novel halostable cellulase from Salinivibrio sp strain NTU-05. Enzyme Microb Tech 44(6–7):373–379. doi:10.1016/j.enzmictec.2009.02.006
Gregg DJ, Boussaid A, Saddler JN (1998) Techno-economic evaluations of a generic wood-to-ethanol process: effect of increased cellulose yields and enzyme recycle. Bioresource Technol 63(1):7–12. doi:10.1016/S0960-8524(97)00103-X
Knauf M, Moniruzzaman M (2004) Lignocellulosic biomass processing: a perspective. Int Sugar J 106(1263):147–150
Park S, Venditti RA, Abrecht DG, Jameel H, Pawlak JJ, Lee JM (2007) Surface and pore structure modification of cellulose fibers through cellulase treatment. J Appl Polym Sci 103(6):3833–3839. doi:10.1002/App.25457
Singhania RR, Sukumaran RK, Pandey A (2007) Improved cellulase production by Trichoderma reesei RUT C30 under SSF through process optimization. Appl Biochem Biotech 142(1):60–70. doi:10.1007/s12010-007-0019-2
Jouenne T, Bonato H, Mignot L, Junter GA (1993) Cell immobilization in composite agar layer microporous membrane structures: growth kinetics of gel-entrapped cultures and cell leakage limitation by a microporous membrane. Appl Microbiol Biotechnol 38(4):478–481
Misro SK, Kumar R, Banerjee R, Bhattacharya BC (1997) Production of gallic acid by immobilization of Rhizopus oryzae. Bioprocess Eng 16:257–260
Galazzo JL, Bailey JE (1990) Growing Saccharomyces cerevisiae in calcium alginate beads induces cell alterations that accelerate glucose conversion to ethanol. Biotechnol Bioeng 36:417–426
Zhang X, Bury S, DiBiasio D, Miller JE (1989) Effects of immobilization on growth, substrate consumption, b-galactosidase induction, and by-product formation in Escherichia coli. J Ind Microbiol 4:239–246
Basu S, Saha MN, Chattopadhyay DJ, Chakrabarti K (2008) Degumming and characterization of ramie fiber using pectate lyase from immobilized Bacillus pumilus DKS1. Lett Appl Microbiol 48:593–597
Mohapatra KDP, Mondal CK, Pati RB (2007) Production of tannase by the immobilized cells of Bacillus licheniformis KBR6 in Ca alginate beads. J Appl Microbiol 102:1462–1467
Kuhad RC, Gupta R, Singh A (2011) Microbial cellulases and their industrial applications. Enzym Res doi:10.4061/2011/280696 (280696:280610)
Cavaco-Paulo A, Gubitz GM (2003) Textile processing with enzymes. Woodhead Publishing, Cambridge
Sreenath HK, Shah AB, Yang VW, Gharia MM, Jeffries TW (1996) Enzymatic polishing of jute/cotton blended fabrics. J Ferment Bioeng 81(1):18–20. doi:10.1016/0922-338x(96)83113-8
Kan CW, Yuen CWM, Jiang SQ (2008) Enzymatic treatment of linen. J Text I 99(4):363–368. doi:10.1080/00405000701442577
Wakarchuk WW, Kilburn DG, Miller RC, Warren RAJ (1986) The molecular-cloning and expression of a cellobiase gene from an agrobacterium in Escherichia coli. Mol Gen Genet 205(1):146–152. doi:10.1007/Bf02428044
Rodriguez-Palenzuela P, Burr TJ, Collmer A (1991) Polygalacturonase is a virulence factor in Agrobacterium tumefaciens biovar 3. J Bacteriol 173(20):6547–6552
Herlache TC, Hotchkiss AT Jr, Burr TJ, Collmer A (1997) Characterization of the Agrobacterium vitis pehA gene and comparison of the encoded polygalacturonase with the homologous enzymes from Erwinia carotovora and Ralstonia solanacearum. Appl Environ Microbiol 63(1):338–346
Stredansky M, Conti E (1999) Succinoglycan production by solid-state fermentation with Agrobacterium tumefaciens. Appl Microbiol Biotechnol 52(3):332–337
Shaw N, Lehner B, Fuhrmann M, Kulla H, Brass J, Birch O, Tinschert A, Venetz D, Venetz VV, Sanchez JC, Tonella L, Hochstrasser D (1999) Biotin production under limiting growth conditions by Agrobacterium/Rhizobium HK4 transformed with a modified Escherichia coli bio operon. J Ind Microbiol Biotechnol 22(6):590–599
Grifantini R, Pratesi C, Galli G, Grandi G (1996) Topological mapping of the cysteine residues of N-carbamyl-D-amino-acid amidohydrolase and their role in enzymatic activity. J Biol Chem 271(16):9326–9331
Carder JH (1986) Detection and quantitation of cellulases by Congo Red staining of substrates in a cup-plate diffusion assay. Anal Biochem 153:73–79
Ghosh A, Maity B, Chakrabarti K, Chattopadhyay D (2007) Bacterial diversity of East Calcutta wet land area: possible identification of potential bacterial population for different biotechnological uses. Microb Ecol 54(3):452–459. doi:10.1007/s00248-007-9244-z
Ghosh A, Dey N, Bera A, Tiwari A, Sathyaniranjan K, Chakrabarti K, Chattopadhyay D (2010) Culture independent molecular analysis of bacterial communities in the mangrove sediment of Sundarban, India. Saline Syst 6(1):1. doi:10.1186/1746-1448-6-1
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739. doi:10.1093/molbev/msr121
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59(2):257–268. doi:10.1351/pac198759020257
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Basu A (2001) Textile testing fibre, yarn and fabric, 1st edn. SITRA, Coimbatore, pp 65–68
Basu S, Saha MN, Chattopadhyay D, Chakrabarti K (2009) Large-scale degumming of ramie fibre using a newly isolated Bacillus pumilus DKS1 with high pectate lyase activity. J Ind Microbiol Biotechnol 36(2):239–245. doi:10.1007/s10295-008-0490-y
Booth JE (1968) Principles of textile testing, 3rd edn. Butterworths, London
Saitou N, Nei M (1986) The neighbor-joining method—a new method for reconstructing phylogenetic trees. Jpn J Genet 61(6):611–612
Felsenstein J (1985) Confidence-limits on phylogenies—an approach using the bootstrap. Evolution 39(4):783–791. doi:10.2307/2408678
Jukes TH, Cantor CR (1969) Evolution of protein molecules. In: Mammalian protein metabolism. Academic Press, New York
Kapoor M, Beg QK, Bhushan B, Dadhich KS, Hoondal GS (2000) Production and partial purification and characterization of a thermo-alkali stable polygalacturonase from Bacillus sp MG-cp-2. Process Biochem 36(5):467–473. doi:10.1016/S0032-9592(00)00238-7
Azevedo H, Bishop D, Cavaco-Paulo A (2000) Effects of agitation level on the adsorption, desorption, and activities on cotton fabrics of full length and core domains of EGV (Humicola insolens) and CenA (Cellulomonas fimi). Enzyme Microb Technol 27(3–5):325–329
Kuhad RC, Kapoor M, Rustagi R (2004) Enhanced production of an alkaline pectinase from Streptomyces sp RCK-SC by whole-cell immobilization and solid-state cultivation. World J Microb Biot 20(3):257–263. doi:10.1023/B:Wibi.0000023833.15866.45
Muyima NY, Zamxaka M, Mazomba NT (2001) Comparative evaluation of pectolytic and proteolytic enzyme production by free and immobilized cells of some strains of the phytopathogenic Erwinia chrysanthemi. J Ind Microbiol Biotechnol 27(4):215–219. doi:10.1038/sj/jim/7000172
Beshay U, Moreira A (2005) Production of alkaline protease with Teredinobacter turnirae in controlled fed-batch fermentation. Biotechnol Lett 27(19):1457–1460. doi:10.1007/s10529-005-1309-9
Acknowledgments
We thank CAS (UGC), DST-FIST for providing some of the instrument facilities at the Department of Biochemistry, University of Calcutta. AB was supported by doctoral fellowship from UGC-SAP-RFSMS scheme. AG is supported by Ramanujan Fellowship from Department of Science and Technology, India (SR/S2/RJN-106/2012).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bera, A., Ghosh, A., Mukhopadhyay, A. et al. Improvement of degummed ramie fiber properties upon treatment with cellulase secreting immobilized A. larrymoorei A1. Bioprocess Biosyst Eng 38, 341–351 (2015). https://doi.org/10.1007/s00449-014-1274-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1274-6