Abstract
Lung diseases occupy a leading position in human morbidity and are the third leading cause of death. Often the chronic forms of these diseases do not respond to therapy, so that lung transplantation is the only treatment option. The development of cellular and biotechnologies offers a new solution—the use of lung organoids for transplantation in such patients. Here, we review types of lung organoids, methods of their production and characterization, and experimental works on transplantation in vivo. These results show the promise of work in this direction. Despite the current problems associated with a low degree of cell engraftment, immune response, and insufficient differentiation, we are confident that organoid transplantation will find it is clinical application.
Similar content being viewed by others
Introduction
Chronic respiratory diseases (CRDs) are one of the main causes of disability and death. According to the Global Burden of Diseases, Injuries, and Risk Factors Study, 544.9 million people worldwide had a CRD in 2017, a 39.8% increase from 1990 (Soriano et al. 2020). As stated by WHO, chronic obstructive pulmonary disease (COPD) was the third leading cause of death in 2019, after ischemic heart disease and stroke. By 2040, premature mortality from many non-communicable diseases, including COPD and lung cancer, will increase by more than 70% by 2040 (Foreman et al. 2018). Oxygen therapy, pulmonary rehabilitation, and pharmacological and surgical treatments (lung volume reduction surgery and lung transplantation) are current strategies for CRD (Keen et al. 2017; Siddiqui et al. 2018). Cell therapy and tissue engineering are modern and actively developing methods for treatment of lung diseases (Geiger et al. 2017; Sun et al. 2018; Kadyk et al. 2017). As of December 2020, ClinicalTrials.gov reported eleven completed clinical trials on transplantation of various types of cells (hematopoietic stem cells, mesenchymal stem cells (MSCs), bone marrow mononuclear cells, bronchial basal cells, and endothelial progenitor cells) for the treatment of lung diseases. Intravenous administration of MSCs was reported to be safe in patients with moderate to severe acute respiratory distress syndrome (ARDS) and COPD (Zheng et al. 2014; Wilson et al. 2015; Matthay et al. 2019; Weiss et al. 2013). Infusion of autologous bone marrow mononuclear cells in patients with COPD was also safe (Stessuk et al. 2013; Ribeiro-Paes et al. 2011). In 2020, a review article was published that summarized clinical data on the feasibility, safety, and tolerability of infusion of MSCs derived from bone marrow or umbilical cord in Severe Acute Respiratory Syndrome, ARDS, and Middle East Respiratory Syndrome (Majolo et al. 2020). Only three stem cell-related clinical trials were considered complete, of which two were in Phase 1 and one was in Phase 2. Cell therapies were shown to cause no complications in gas exchange, spirometry, quality of life, cardiopulmonary circulation, and immune system of those suffering from the lung disease. However, there exist some obstacles such as low mobilization of transplanted MSCs at the site of injury and their low survival rate.
Lung transplantation is often the last chance for patients with various lung conditions. Recently, a new method of transplantation was developed: the lungs undergo decellularization following recellularization (Peloso et al. 2015; Tsuchiya et al. 2014; Tebyanian et al. 2019; Calle et al. 2017). Recellularization is carried out using various types of cells, for example, alveolar epithelial cells, endothelial cells, MSCs, embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and other stem/progenitor cells. Currently, decellularization protocols are being standardized and recellularization methods are being optimized.
The use of three-dimensional (3D) cell structures, such as organoids, is a new and dynamically developing direction in cell therapy. Organoids, according to Huch and Koo, are defined as a 3D structure derived from either PSCs, neonatal tissue stem cells, or adult progenitor cells, in which cells spontaneously self-organize into properly differentiated functional cell types and progenitors, and which resemble their in vivo counterparts and recapitulate at least some of the functions of the organ (Huch et al. 2015). Organoids are used for study of intercellular interactions, disease modeling, drug screening, and regenerative medicine (Nikolić et al. 2017b; Kim et al. 2020; Bartfeld et al. 2017). Lung organoids recapitulate lung development and may serve as a useful tool for modeling lung disease (Chen et al. 2017a, b). In this review, we shall discuss the main type of lung organoids and how to obtain them. We shall also focus on in vivo lung organoid transplantation studies to analyze the viability, engraftment, and maturation of organoids, as well as the effectiveness of the treatment of pulmonary diseases.
Types of lung organoids
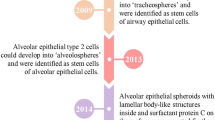
The respiratory system consists of the upper and lower respiratory tracts. The upper respiratory tract comprises the nasal cavity, pharynx, and larynx. The lower respiratory tract consists of the trachea, primary bronchi leading to the bronchioles, and alveoli. The epithelium in the lower respiratory tract is mainly composed of basal cells, goblet cells producing mucus, and ciliated cells required for mucociliary clearance (Chang et al. 2008; Klein et al. 2011). In the bronchioles, the epithelium consists of more cuboidal-shaped cells with shorter cilia and secretory club cells; in the alveoli, the epithelium consists of alveolar type I and II cells. Alveolar type II cells perform the functions of surfactant producers or differentiate into alveolar type I cells. Thus, the most common classification of lung organoids is based on the cell types present in organoids (Fig. 1):
-
airway organoids (include tracheospheres, bronchospheres, and nasospheres) consisting of ciliated, goblet, basal, club, tuft, and pulmonary neuroendocrine cells;
-
alveolar organoids consisting of alveolar type I and II cells;
-
lung organoids consisting of cell types characteristic of both airway and alveolar organoids.
Airway, alveolar, and lung organoids can be obtained from both adult cells and pluripotent stem cells, including ESCs and iPSCs (Table 1).
Airway organoids
Airway organoids derived from adult cells are often classified according to the location of the biopsy used to isolate these cells, namely, tracheospheres, bronchospheres, and nasospheres. Such organoids are characterized by the expression of markers of basal cells (KRT8 + , KRT14 + , and p63 +), ciliated cells (AC-TUB + and FOXJ1 +), mucosecretory or goblet cells (MUC5AC + and MUC5B +), and club cells (CC10 +) (Rock et al. 2009; Lee et al. 2020; Tesei et al. 2009; Kumar et al. 2011).
All protocols for obtaining airway organoids from adult cells are similar to each other with some variations (Fig. 2). The first protocol for obtaining mouse tracheospheres was published in 2009 and involved mechanical separation and enzymatic digestion of a piece of mouse trachea, fluorescence-activated cell sorting (FACS), 1:1 mixing with Matrigel and further cultivating on the Transwell inserts at the air–liquid interface (ALI) for 26 days (Rock et al. 2009). The ALI-culture method consists of seeding cells onto a permeable membrane of a cell culture insert, with the basal side of the cells in contact with liquid culture medium, whereas the apical side is exposed to air. This initiates the differentiation of cells into a mature polarized pseudo-stratified epithelium consisting of functional basal, ciliated, and secretory cells (Choi et al. 2020; Kumar et al. 2011; Usui et al. 2000). Another method called culturing self-assembled spheres (SAS) uses seeding of cells after their digestion onto ultra-low attachment plates (Tesei et al. 2009). With nasal spheroids, ALI cultures were shown to stratify into basal cells and suprabasal differentiated cells, while SAS cultures remain a monolayer of ciliated and goblet cells and lack basal cells (Kumar et al. 2011). And one of the most popular methods of organoid cultivation is the use of the extracellular matrix. One of the most common matrices for cultivation is Matrigel. Matrigel is xenogenic; it is derived from the basement membrane matrix secreted by Engelbreth–Holm–Swarm mouse sarcoma cells. Matrigel is extremely complex: it contains more than 1,800 unique proteins (Hughes et al. 2010). The concentrations of growth factors and mechanical and biochemical properties may vary from batch to batch in Matrigel, which may lead to uncertainty in cell culture experiments and lack of reproducibility (Aisenbreyet al. 2020). Many synthetic matrices based on polyacrylamide and polyethylene glycol, as well as natural matrices based on decellularized tissues, are currently being developed (Aisenbreyet al. 2020; Kozlowski et al. 2021).
Airway organoids from embryonic stem cells are obtained in the same way as from adult cells. Nikolić M. Z. et al. showed that human embryonic lung distal tip epithelium comprises a multipotent progenitor cell population with the capacity of self-renewal and differentiation (Nikolić et al. 2017a). Human lung epithelial tips from 5 to 9 weeks post-conception were microdissected, digested, mixed with Matrigel, and transferred into a low-attachment plate. Then, the tip-derived organoids were differentiated into bronchiolar and alveolar lineages. The bronchiolar organoids at high passages contained mostly goblet cells (MUC5AC +), while at lower passages, the organoids contained basal cells (KRT5 +).
The development of the lungs consists of the following stages: embryonic (appearance of the definitive and anterior foregut endoderm), pseudoglandular (formation of the bronchial tree), canalicular (expansion of the respiratory tree), saccular (specification of the alveolar epithelium), and alveolar (maturation of the alveoli and microvessels). During embryogenesis, the definitive endoderm (DE) develops and transforms into a gut tube located along the anterior–posterior and dorsal–ventral axes (Zorn et al. 2009). As the development proceeds, the anterior foregut endoderm (AFE) is formed, which gives rise to the esophagus, trachea, stomach, lungs, thyroid, liver, biliary system, and pancreas. Then, lung specification begins with the expression of the thyroid transcription factor-1 (NKX2.1) on the ventral side of the AFE (Goss et al. 2009). Thus, obtaining airway organoids from iPSCs includes differentiation into DE, then into AFE, and finally into NKX2-1 + lung epithelial progenitors which are purified by sorting cell surface markers and cultured in Matrigel with WNT signaling inhibitors to form 3D epithelial-only airway organoids, since McCauley K. B. et al. showed that the inhibition of WNT signaling efficiently induces proximal lung progenitors, while the activation of WNT leads to a significant increase in distal patterning (McCauley et al. 2017, 2018). The airway organoids obtained using this protocol contain all cells characteristic of this type of organoids (basal, ciliated, goblet, and club cells). Figure 3 summarizes different protocols for differentiation of iPSCs into airway, alveolar, and lung organoids.
Alveolar organoids
Alveolar organoids can be derived from adult tissue, embryonic progenitors, and induced pluripotent stem cells; they are characterized by the expression of markers of AT2 cells (SFTPC + , ProSPC + , AQP5 + , HT2-280 +) and alveolar type 1 (AT1) cells. In research, AT2 cells are isolated from adult or embryonic tissues, since these cells behave as facultative stem cells, they are capable of self-renewal and differentiation into AT1 cells (Evans et al. 2020). For isolation of AT2 cells, it is necessary to enzymatically digest and mechanically dissociate whole lungs or transbronchial samples (Barkauskas et al. 2013, 2017; Dinh et al. 2017; Nikolić et al. 2017a; Zacharias et al. 2018). Dissociated AT2 cells are isolated by FACS (EPCAM + , HT2‐280 + or TM4SF1-, APC +), mixed with fibroblasts and Matrigel and ALI cultured (Zacharias et al. 2018). Dinh P. U. C. suggested an alternative protocol according to which tissue explants are cultured on a fibronectin-coated plate for 17–25 days (Dinh et al. 2017). After that, cells are seeded into an ultra-low attachment flask and in 5–7 days reseeded onto fibronectin-coated surfaces to produce alveolar organoids. Recent studies showed that alveolar organoids derived from both murine and human AT2 cells are capable of differentiating into AT1 cells in vitro (Chen et al. 2021; Katsura et al. 2020). Obtaining alveolar organoids containing AT1 and AT2 cells from iPSCs is reduced to the differentiation of NKX2.1 + cells using a combination of CHIR99021, FGF10, KGF, and DAPT, which are plated in Matrigel and cultured with or without lung fibroblasts. Co-cultivation with fibroblasts proved to be advantageous for the induction and stable expansion of SFTPC + cell populations while maintaining their stem cell properties, which suggests that the niche provided by epithelial–mesenchymal interaction may be crucial for maintaining the progenitor properties of AT2 cells (Yamamoto et al. 2017; Kim et al. 2021).
Lung organoids
Lung organoids can also be derived from adult tissue, embryonic progenitors, induced pluripotent stem cells; they contain proximal and distal lung cells, such as basal, ciliated, club, AT1, and AT2 cells. Obtaining lung organoids from adult tissue consists of enzymatic digestion and 3D culturing (Nikolić et al. 2017a). Both for organoids derived from iPSCs and for organoids derived from embryonic cells, a similar protocol of differentiation into lung organoids is employed (Leibel et al. 2020). Leibel et al. described a protocol for the production of human lung organoids (HLOs) from PSCs by endoderm induction using activin A and CHIR99021, followed by induction of anterior foregut endoderm using inhibition of BMP and TGFβ signaling by SB431542 and dorsomorphin (Leibel et al. 2020). The anterior foregut cells were then cultured with BMP4, CHIR99021, and all‐trans retinoic acid (ATRA). At the last stage, the cells were placed in Matrigel with FGF7, FGF10, EGF, and CHIR99021 to form organoids. The resulting organoids expressed airway (KRT5 + , MUC5AC + , SCGB3A2 +) and alveolar (SP‐C + , SP‐B + , HTII‐280 + , AGER +) markers. Dye et al. described a similar protocol for obtaining lung organoids from iPSCs, however, the authors obtained anterior foregut spheroids which were then placed in Matrigel with Noggin, SB431542, FGF4, CHIR99021, and 1% fetal bovine serum for maturation of lung organoids (Dye et al. 2015). The resulting organoids expressed proximal (SOX2 +) and distal (SOX9 + , HOPX + , SFTPC +) lung markers. HLOs cultured for more than 2 months had epithelial structures resembling the proximal and distal airways and expressing markers of basal (P63 +), ciliated (FOXJ1 + , AC-TUB +), club (SCGB1A1 +), AT2 (SFTPC + , SFTPB +), and AT1 (PDPN + , HOPX +) cells.
Transplantation of lung organoids for maturation
Almost all of the above described organoids that were obtained in vitro have incomplete differentiation so far as they are not exposed to air (Schilders et al. 2016). Therefore, in some of the studies, the last stage of differentiation is transplantation which promotes cell maturation and formation of vascular and neuronal networks (Tan et al. 2017; Chen et al. 2018). It is also worth noting that «organoid transplantation» refers to both the transplantation of whole organoids and the transplantation of cell suspensions derived from organoids.
The most common in vivo approach is xenotransplantation, in which human organoids are transplanted into laboratory animals. Many studies showed that human lung organoids can be grafted to mammalian hosts. Dinh et al. successfully engrafted human lung spheroids into athymic nude mice; on days 1, 4, 7, 11, and 20 after transplantation, cells were found in the lungs and liver (Dinh et al. 2017). The cells expressed alveolar (AQP5 + and ProSPC +), secretory (CCSP +), and epithelial (EPCAM +) markers in the lung tissue, while they were absent in the liver. Thus, the authors showed that lung spheroid cells engrafted into mammalian hosts retain lung phenotype in orthotopic transplantation, in contrast to ectopic transplantation.
Orthotopic transplantation has important advantages, such as providing the best environment for cell survival and function, allowing to assess vascularization and neurogenesis, increasing the translational potential of organoid models, etc. However, in most cases, lung organoids are transplanted into an ectopic site, since this opens up the possibility of choosing a site with good access to blood supply and maintenance of vascularization of the grafted tissue without impairing the necessary functions of mammalian organ (Deward et al. 2014; Holloway et al. 2019). The site of transplantation was shown to have no effect on the engraftment of organoids (Dye et al. 2016). The authors transplanted HLOs grown in vitro from 1 to 65 days into the subcapsular pocket of the mouse kidney and into the greater omentum. They showed that alien sites of ectopic localization do not reliably support the survival or growth of the HLO lung epithelium in vivo.
Unlike ectopic localization, orthotopic graft localization provides the best environment for the cell and plays an important role in the maturation and differentiation of organoids. Chen et al. hypothesized that ectopic localization prevents organoids from definitive differentiation (Chen et al. 2017b). Human lung bud organoids (LBOs) were transplanted under the kidney capsule into immunodeficient NSG mice. After 7 months, LBOs were found at the transplantation site, but full phenotypic and architectural alveolar maturation was not achieved. However, (Nikolić et al. 2017a) showed (also in 2017) that 8 days after orthotopic in vivo transplantation of lung tip organoids (SOX2 + , SOX9 +) into NGS mice, cells retained the co-expression of SOX2 and SOX9, which means incomplete airway differentiation (Nikolić et al. 2017a). This result can be explained by the short observation period. The authors then performed an ectopic transplantation of lung tip organoids that were dissociated and mixed with a cell pellet formed from dissociated E13.5 mouse lungs under the kidney capsule of NSG mice. After 12 weeks, only some human cells expressed SOX9 at a very low level; thus, alveolar differentiation was either premature or ineffective. The authors concluded that human cells may be unable to respond efficiently to mouse differentiation signals, possibly because they require different signaling inputs.
As mentioned above, transplantation is in most cases considered the last stage in the differentiation of lung organoids, since lung organoids differentiate better in vivo than in vitro. The authors compared the efficiency of differentiation of HLOs grown in vitro and HLOs seeded onto a bioartificial microporous poly(lactide-co-glycolide) (PLG) scaffold, cultured for 5–7 days in vitro and transplanted into epididymal fat pads of mice (Dye et al. 2016). The use of PLG scaffold with HLOs demonstrated that 8 weeks after transplantation 100% of the recovered constructs possessed huMITO + and NKX2.1 + mature airway-like structures, while in the control group (HLOs in a Matrigel plug), no tissue recovery was observed. In addition, 8 weeks after transplantation, multiple epithelial structures per cross section were observed. The identified airway-like structures were densely surrounded by mesenchymal cells and there were pockets of organized cartilage throughout the transplants, while HLOs seeded on the scaffold and grown in vitro for 4–8 weeks did not represent epithelial structure. Therefore, the authors concluded that the scaffold provides critical support for the engraftment and survival of the lung epithelium and the combination of the scaffold and the in vivo environment ensures the growth and maturation of the HLO epithelium. Later, in a 2019 study, the authors showed that the rate of scaffold degradation affects the HLO maturation—rapidly degrading polymers lead to an increase in the size of airway structures (Dye et al. 2020). The use of scaffolds in research is advancing the field of regenerative medicine, where matrices play an important role and should provide a stable and supportive vehicle to deliver cells to the desired location in vivo (Aisenbrey et al. 2020). Matrigel, due to its animal origin, makes it difficult to use it in the future in human transplantation in clinic due to potential immunogenicity (Schneeberger et al. 2017; Kozlowski et al. 2021). therefore, the creation of suitable matrices is an important direction for future research.
It was also shown that the stage of transplanted HLO culture did not affect the survival of the HLO lung epithelium (Dye et al. 2016). However, in (Chen et al. 2018) transplanted HLOs at different stages of cultivation into the subcapsular pocket of the kidney of immunodeficient B-NSG mice and showed that it is better to use 41-day HLOs to obtain mature AT1-like cells and AT2 cells after long-term transplantation, since at this time the expression of the genes of distal cells of the alveolar epithelium reaches maximum. Earlier, on days 21 and 31 of culturing HLOs in vitro, there is a peak in the expression of the genes specific for lung progenitors and stem cells (NKX2.1, SOX9, SOX2, and P63) and transplanted HLOs can differentiate into bipotent progenitor cells after long-term engraftment (Chen et al. 2018). The authors also showed that 100–120 days after transplantation HLOs possessed a vascular network (ACTA2 +) and a neuronal network (PGP9.5 +), resembling the human lung.
The issue of vascularization is important in transplantation, since it is critical to ensure the distribution of oxygen and nutrients in large organoids in vivo and to provide the integration of airway organoid grafts with the host tissue (Tan et al. 2017; Vargas-Valderrama et al. 2020). In (Tan et al. 2017) obtained multicellular airway organoids containing vascular structures by combining human primary adult bronchial epithelial cells with human primary adult microvascular lung endothelial cells and human primary adult lung fibroblasts at a ratio of 10:7:2; they were transplanted under the kidney capsule of NSG mice (Tan et al. 2017). One week after implantation, organoids were visible in the kidney capsule, with human specific CD31 + endothelial cells within airway organoids. However, staining with specific anti-human antibodies demonstrated that the vast majority of proliferating cells was not within airway organoids, but rather within host tissue. Six weeks after implantation, the organoids regressed in size, abundant host vasculature invaded the organoid area where proximal secretory airway cells (CC10 +) and distal alveolar cells (AQP5 + and SPC +) were observed. Thus, the authors showed that bronchial epithelial, mesenchymal, and vascular cells in the airway organoids can survive and undergo significant maturation after engraftment in vivo; however, ectopic transplantation, as mentioned earlier, prompted a shift towards cell lineage commitment and differentiation towards mature, non-proliferating states, limiting the regenerative potential of the current system.
Transplantation of lung organoids for repair of lung injury
Model of lung injury in mice can be created by direct damage to the bronchoalveolar epithelium and capillary endothelium due to intratracheal administration of acid or bleomycin, prolonged hyperoxia, prolonged mechanical ventilation at high tidal volume, or intravenous injection of oleic acid or endotoxin (lipopolysaccharide) (Aeffner et al. 2015). Ischemia/reperfusion models, sepsis models, influenza virus models, and secondary peritonitis models can also be used. ‘Smoking mouse’ models accurately reflect the pathophysiology of COPD, since cigarette smoke is the main cause of this disease (Vlahos et al. 2014). There are several works on transplantation of lung organoids into the area of lung injury (Nikolić et al. 2017a; Miller et al. 2018; Weiner et al. 2019). In 2015, sublethal whole-body irradiation of the recipient before the transplantation of cells was developed (Rosen et al. 2015). Sublethal irradiation was performed 48 h after naphthalene treatment to clear the precursor niches in the lungs, reduce the competition of stem cells and improve engraftment of infused donor cells. It was shown that exposure to irradiation before treatment with naphthalene did not lead to effective destruction of the endogenous pool of precursors. Although the authors transplanted human fetal lung tissue and not organoids, we consider this study to be a key publication on the long-term engraftment of transplanted lung cells and organoids. The need for irradiation to engraft the lungs with transplanted cells is the most significant obstacle for the clinical development and implementation of this approach. The search for other ways to eliminate recipient’s own stem cells in the appropriate niches is necessary for the development of organoid transplantation (Table 2). Although numerous studies show that irradiation is not necessary, the efficiency of cell engraftment after irradiation is significantly improved.
In addition to the competition of stem cells, there is the problem of immune rejection in allotransplantation, which is usually solved by chronic suppression of the immune system. Hillel-Karniel et al. developed a protocol that allowed efficient transplantation across major genetic barriers by co-infusion of T cell-depleted hematopoietic progenitor cells together with lung cells and treatment with cyclophosphamide after transplantation (Hillel-Karniel et al. 2020). On the other hand, obtaining organoids from donor’s own iPSCs eliminates the problem of immune rejection, which is a great advantage.
An analysis of the literature shows that human organoids can be transplanted into mice, and after a few days or weeks human cells can be detected. In 2018, transplantation of bud tip progenitor organoids derived from hPSCs into the airways of injured mouse lungs was performed (Miller et al. 2018). A short-term engraftment experiment showed that 79% of the cells expressed the markers SOX2 and SOX9, half of the cells expressed the club cell marker SCGB1A1 and a quarter of the cells expressed the goblet cell marker MUC5AC; no other cell markers were observed. Three groups of mice were involved in the long-term engraftment experiment: (a) received an injury but no injection of cells; (b) received an injury and injection of undifferentiated hPSCs; and (c) received an injury and injection of bud tip organoid cells. Lungs in animals of all experimental groups successfully recovered from the injury. Engraftment of human cells was observed in 8 out of 15 surviving mice that received bud tip organoid injections. Engrafted cells were found in bronchioles, trachea, and primary/secondary bronchi of mice. All engrafted human cells expressed SOX2, with roughly 75% of the cells acquiring a mucus-producing phenotype, ∼13% acquiring a ciliated cell profile, and ∼0.5% exhibiting a neuroendocrine cell profile. The P63 marker or alveolar cell specific markers were not detected. In 2019, AT2 organoids and primary AT2 cells were transplanted into influenza-injured recipient mice 11 days after infection (Weiner et al. 2019). Mice with cellular transplantation recovered ~ 65% faster than the control group, whereas the transplantation of AT2 organoids did not improve the recovery process. Thirteen days after transplantation, cells from AT2 organoids demonstrated two distinct fates: maintenance of AT2 lineage (SPC + , Lamp3 +) or dysplastic regeneration (Scgb3a2 + , Krt5 +), while primary AT2 cells either retained their AT2 lineage or differentiated into AT1 cells and did not exhibit dysplastic regeneration. The authors concluded that primary AT2 cells which never experienced in vitro conditions may retain a more appropriate lineage restriction upon transplantation.
Another significant problem in the transplantation of organoids derived from embryonic or induced pluripotent stem cells is incomplete differentiation and presence of poorly differentiated cells in the cellular mass that could theoretically form tumors (teratomas, for example) after transplantation. To date, this problem has been insufficiently studied. It is necessary to study the long-term consequences of transplantation of stem cell-derived organoids not only from the point of view of the survival rate of donor cells, but also for the assessment of the risk of tumor formation. In addition, it is necessary to develop protocols for detecting incomplete differentiation of stem cells before transplantation.
Lung organoids are promising instruments in regenerative medicine, since organoids can be obtained from a small amount of donor cells and can provide autologous cells or even tissue for transplantation (Bartfeld et al. 2017). Lung organoids are believed to be grafted onto scaffolds, including a decellularized lung, as it is done today with gastric, hepatic, pancreatic, and small intestinal organoids (Giobbe et al. 2019). Various methods of scaffold grafting with cells and organoids have been reported. For each of these methods, successful growth and maturation of cells were observed, which gives hope for their further development and application to lung organoids.
Organoid technology can be combined with recent advances in genome editing based on CRISPR-Cas9. There are three ways to incorporate CRISPR-Cas9 method in organoid technology: genome modification of cells prior to creation of organoids; genome modification of organoids dissociated into single cells and subsequent re-formation of 3D structures; delivery of Cas9 and sgRNA to the organoids without their dissociation into single cells (Gopal et al. 2020). The first two methods are often used in the studies of lung diseases, including generation of disease-specific lung organoids (Strikoudis et al. 2019), or for the correction of the CFTR gene in intestinal organoids (Maule et al. 2020).
Conclusion
Lung organoids are a promising approach in the treatment of pulmonary diseases. The review describes the main protocols for obtaining airway, alveolar, and lung organoids from adult tissue, embryonic progenitors, and induced pluripotent stem cells. Organoids are transplanted into ectopic and orthotopic sites for maturation and formation of vascular and neuronal networks. In most cases, lung organoids are transplanted into an ectopic site, since this opens up the possibility of choosing a site with good access to blood supply and maintenance of vascularization of the grafted tissue; however, the best results of organoid maturation were obtained with orthotopic transplantation. Studies on the transplantation of lung organoids into the area of lung injury showed that organoids engraft, preserve the phenotype, and contribute to the repair of injured tissue. However, there is little research on the fate of transplanted organoids, their vascularization and restoration of tissue architecture over a long period of time.
Since organoids have appeared relatively recently, their use in clinical practice requires their thorough characterization, development of the most effective protocols for obtaining and transplantation, and conductance of preclinical studies. In our opinion, there are still a number of unresolved problems in this area. It is necessary to develop protocols for confirming complete differentiation of stem cells, if they are the source of organoids. Protocols for successful colonization of the lungs with donor cells need to be improved, as irradiation is dangerous for patients. Solving these problems will bring us closer to the clinical use of respiratory organoids for the treatment of various lung diseases.
References
Aeffner F, Bolon B, Davis IC (2015) Mouse models of acute respiratory distress syndrome: a review of analytical approaches, pathologic features, and common measurements. Toxicol Pathol 8:1074–1092. https://doi.org/10.1177/0192623315598399
Aisenbrey EA (2020) Murphy WL (2020) Synthetic alternatives to Matrigel. Nat Rev Mater 57(5):539–551. https://doi.org/10.1038/s41578-020-0199-8
Barkauskas CE, Chung MI, Fioret B, Gao X, Katsura H, Hogan BLM (2017) Lung organoids: current uses and future promise. Dev 144:986–997
Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BLM (2013) Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 123:3025–3036. https://doi.org/10.1172/JCI68782
Bartfeld S, Clevers H (2017) Stem cell-derived organoids and their application for medical research and patient treatment. J Mol Med 95:729–738
Calle EA, Leiby KL, Raredon MB, Niklason LE (2017) Lung regeneration: steps toward clinical implementation and use. Curr Opin Anaesthesiol 30:23–29
Chang MMJ, Shih L, Wu R (2008) Pulmonary epithelium: cell types and functions. Pulm Ep Heal Dis 1–26. https://doi.org/10.1002/9780470727010.CH1
Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A, Wei Y, Vu TH (2011) Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest 121:2855–2862. https://doi.org/10.1172/JCI57673
Chen Q, Liu Y (2021) Isolation and culture of mouse alveolar type II cells to study type II to type I cell differentiation. STAR Protoc 2:100241. https://doi.org/10.1016/J.XPRO.2020.100241
Chen Y-W, Ahmed A, Snoeck H-W (2017a) Generation of three-dimensional lung bud organoid and its derived branching colonies. Protoc Exch. https://doi.org/10.1038/protex.2017.027
Chen Y, Feng J, Zhao S, Han L, Yang H, Lin Y, Rong Z (2018) Long-term engraftment promotes differentiation of alveolar epithelial cells from human embryonic stem cell derived lung organoids. Stem Cells Dev 27:1339–1349. https://doi.org/10.1089/scd.2018.0042
Chen YW, Huang SX, De Carvalho ALRT, Ho SH, Islam MN, Volpi S, Notarangelo LD, Ciancanelli M, Casanova JL, Bhattacharya J, Liang AF, Palermo LM, Porotto M, Moscona A, Snoeck HW (2017b) A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat Cell Biol 19:542–549. https://doi.org/10.1038/ncb3510
Choi KYG, Wu BC, Lee AHY, Baquir B, Hancock RE (2020) Utilizing organoid and air-liquid interface models as a screening method in the development of new host defense peptides. Front Cell Infect Microbiol 10:228. https://doi.org/10.3389/FCIMB.2020.00228/BIBTEX
Deward AD, Komori J, Lagasse E (2014) Ectopic transplantation sites for cell-based therapy. Curr Opin Organ Transplant 19:169–174
Dinh P-UC, Cores J, Hensley MT, Vandergriff AC, Tang J, Allen TA, Caranasos TG, Adler KB, Lobo LJ, Cheng K (2017) Derivation of therapeutic lung spheroid cells from minimally invasive transbronchial pulmonary biopsies. Respir Res 18:132. https://doi.org/10.1186/s12931-017-0611-0
Dye BR, Dedhia PH, Miller AJ, Nagy MS, White ES, Shea LD, Spence JR (2016) A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids. Elife 5. https://doi.org/10.7554/eLife.19732
Dye BR, Hill DR, Ferguson MA, Tsai YH, Nagy MS, Dyal R, Wells JM, Mayhew CN, Nattiv R, Klein OD, White ES, Deutsch GH, Spence JR (2015) In vitro generation of human pluripotent stem cell derived lung organoids. Elife 2015:1–25. https://doi.org/10.7554/eLife.05098
Dye BR, Youngblood RL, Oakes RS, Kasputis T, Clough DW, Spence JR, Shea LD (2020) Human lung organoids develop into adult airway-like structures directed by physico-chemical biomaterial properties. Biomaterials 234. https://doi.org/10.1016/j.biomaterials.2020.119757
Evans KV, Lee J-H (2020) Alveolar wars: the rise of in vitro models to understand human lung alveolar maintenance, regeneration, and disease. Stem Cells Transl Med 9:867–881. https://doi.org/10.1002/SCTM.19-0433
Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, Pletcher MA, Smith AE, Tang K, Yuan CW, Brown JC, Friedman J, He J, Heuton KR, Holmberg M, Patel DJ, Reidy P, Carter A, Cercy K, Chapin A, Douwes-Schultz D, Frank T, Goettsch F, Liu PY, Nandakumar V, Reitsma MB, Reuter V, Sadat N, Sorensen RJD, Srinivasan V, Updike RL, York H, Lopez AD, Lozano R, Lim SS, Mokdad AH, Vollset SE, Murray CJL (2018) Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 392:2052–2090. https://doi.org/10.1016/S0140-6736(18)31694-5
Geiger S, Hirsch D, Hermann FG (2017) Cell therapy for lung disease. Eur Respir Rev 26. https://doi.org/10.1183/16000617.0044-2017
Giobbe GG, Crowley C, Luni C, Campinoti S, Khedr M, Kretzschmar K, De Santis MM, Zambaiti E, Michielin F, Meran L, Hu Q, van Son G, Urbani L, Manfredi A, Giomo M, Eaton S, Cacchiarelli D, Li VSW, Clevers H, Bonfanti P, Elvassore N, De Coppi P (2019) Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat Commun 10:1–14. https://doi.org/10.1038/s41467-019-13605-4
Gkatzis K, Panza P, Peruzzo S, Stainier DYR (2021) Differentiation of mouse fetal lung alveolar progenitors in serum-free organotypic cultures. Elife 10. https://doi.org/10.7554/ELIFE.65811
Gopal S, Rodrigues AL, Dordick JS (2020) Exploiting CRISPR Cas9 in three-dimensional stem cell cultures to model disease. Front Bioeng Biotechnol 8:692
Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Min LuM, Yamaguchi TP, Morrisey EE (2009) Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell 17:290–298. https://doi.org/10.1016/J.DEVCEL.2009.06.005
Gotoh S, Ito I, Nagasaki T, Yamamoto Y, Konishi S, Korogi Y, Matsumoto H, Muro S, Hirai T, Funato M, Mae S-I, Toyoda T, Sato-Otsubo A, Ogawa S, Osafune K, Mishima M (2014) Generation of alveolar epithelial spheroids via isolated progenitor cells from human pluripotent stem cells. Stem Cell Reports 3:394–403. https://doi.org/10.1016/J.STEMCR.2014.07.005
Hild M, Jaffe AB (2016) Production of 3-D airway organoids from primary human airway basal cells and their use in high-throughput screening. Curr Protoc Stem Cell Biol 37:IE.9.1-IE.9.15. https://doi.org/10.1002/CPSC.1
Hillel-Karniel C, Rosen C, Milman-Krentsis I, Orgad R, Bachar-Lustig E, Shezen E, Reisner Y (2020) Multi-lineage lung regeneration by stem cell transplantation across major genetic barriers. Cell Rep 30:807-819.e4. https://doi.org/10.1016/j.celrep.2019.12.058
Holloway EM, Capeling MM, Spence JR (2019) Biologically inspired approaches to enhance human organoid complexity. Dev. 146
Huch M, Koo B-K (2015) Modeling mouse and human development using organoid cultures. Development 142:3113–3125. https://doi.org/10.1242/DEV.118570
Hughes CS, Postovit LM, Lajoie GA (2010) Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10:1886–1890. https://doi.org/10.1002/PMIC.200900758
Jacob A, Morley M, Hawkins F et al (2017) Differentiation of human pluripotent stem cells into functional lung alveolar epithelial cells. Cell Stem Cell 21:472-488.e10. https://doi.org/10.1016/J.STEM.2017.08.014
Kadyk LC, DeWitt ND, Gomperts B (2017) Proceedings: regenerative medicine for lung diseases: a CIRM Workshop Report. In: Stem Cells Translational Medicine. John Wiley and Sons Ltd., pp 1823–1828
Katsura H, Sontake V, Tata A, Kobayashi Y, Edwards CE, Heaton BE, Konkimalla A, Takanori Asakura Yu, Mikami EJ, Fritch PJ, Lee NS, Heaton RC, Boucher SH, Randell RS, Baric PR, Tata, (2020) Human lung stem cell-based alveolospheres provide insights into SARS-CoV-2-mediated interferon responses and pneumocyte dysfunction. Cell Stem Cell 27:890-904.e8. https://doi.org/10.1016/J.STEM.2020.10.005
Keen C, Medarov BI (2017) Current strategies in chronic obstructive pulmonary disease management. J Public Health Emerg 1:26
Kim J-H, An GH, Kim J-Y, Rasaei R, Kim WJ, Jin X, Woo D-H, Han C, Yang S-R, Kim J-H (2021) Hong S-H (2021) Human pluripotent stem cell-derived alveolar organoids for modeling pulmonary fibrosis and drug testing. Cell Death Discov 71(7):1–12. https://doi.org/10.1038/s41420-021-00439-7
Kim J, Koo BK, Knoblich JA (2020) Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 21:571–584. https://doi.org/10.1038/s41420-021-00439-7
Klein SG, Hennen J, Serchi T, Blömeke B, Gutleb AC (2011) Potential of coculture in vitro models to study inflammatory and sensitizing effects of particles on the lung. Toxicol in Vitro 25:1516–1534. https://doi.org/10.1016/J.TIV.2011.09.006
Kozlowski MT, Crook CJ (2021) Ku HT (2021) Towards organoid culture without Matrigel. Commun Biol 41(4):1–15. https://doi.org/10.1038/s42003-021-02910-8
Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, Wang DY, Lim B, Chow VT, Crum CP, Xian W, McKeon F (2011) Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 147:525–538. https://doi.org/10.1016/j.cell.2011.10.001
Laube M, Pietsch S, Pannicke T, Thome UH, Fabian C (2021) Development and functional characterization of fetal lung organoids. Front Med 8:1525. https://doi.org/10.3389/FMED.2021.678438/BIBTEX
Lee RE, Miller SM, Mascenik TM, Lewis CA, Dang H, Boggs ZH, Tarran R, Randell SH (2020) Assessing human airway epithelial progenitor cells for cystic fibrosis cell therapy. Am J Respir Cell Mol Biol 63:374–385. https://doi.org/10.1165/rcmb.2019-0384OC
Leibel SL, McVicar RN, Winquist AM, Niles WD, Snyder EY (2020) Generation of complete multi−cell type lung organoids from human embryonic and patient-specific induced pluripotent stem cells for infectious disease modeling and therapeutics validation. Curr Protoc Stem Cell Biol 54. https://doi.org/10.1002/cpsc.118
Majolo F, da Silva GL, Vieira L, Timmers LFSM, Laufer S, Goettert MI (2020) Review of trials currently testing stem cells for treatment of respiratory diseases: facts known to date and possible applications to COVID-19. Stem Cell Rev. Reports 1
Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP, Bajwa EK, Donahoe MP, McVerry BJ, Ortiz LA, Exline M, Christman JW, Abbott J, Delucchi KL, Caballero L, McMillan M, McKenna DH, Liu KD (2019) Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med 7:154–162. https://doi.org/10.1016/S2213-2600(18)30418-1
Maule G, Arosio D, Cereseto A (2020) Gene therapy for cystic fibrosis: progress and challenges of genome editing. Int J Mol Sci 21:1–13
McCauley KB, Hawkins F, Kotton DN (2018) Derivation of epithelial-only airway organoids from human pluripotent stem cells. Curr Protoc Stem Cell Biol 45. https://doi.org/10.1002/cpsc.51
McCauley KB, Hawkins F, Serra M, Thomas DC, Jacob A, Kotton DN (2017) Efficient derivation of functional human airway epithelium from pluripotent stem cells via temporal regulation of Wnt signaling. Cell Stem Cell 20:844-857.e6. https://doi.org/10.1016/j.stem.2017.03.001
Miller AJ, Hill DR, Nagy MS, Aoki Y, Dye BR, Chin AM, Huang S, Zhu F, White ES, Lama V, Spence JR (2018) In vitro induction and in vivo engraftment of lung bud tip progenitor cells derived from human pluripotent stem cells. Stem Cell Reports 10:101–119. https://doi.org/10.1016/j.stemcr.2017.11.012
Mondrinos MJ, Jones PL, Finck CM, Lelkes PI (2014) Engineering de novo assembly of fetal pulmonary organoids. Tissue Eng - Part A 20:2892–2907. https://doi.org/10.1089/ten.tea.2014.0085
Nikolić MZ, Caritg O, Jeng Q, Johnson JA, Sun D, Howell KJ, Brady JL, Laresgoiti U, Allen G, Butler R, Zilbauer M, Giangreco A, Rawlins EL (2017a) Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. Elife 6. https://doi.org/10.7554/eLife.26575
Nikolić MZ, Rawlins EL (2017b) Lung organoids and their use to study cell-cell interaction. Curr Pathobiol Rep 5:223–231
Peloso A, Dhal A, Zambon JP, Li P, Orlando G, Atala A, Soker S (2015) Current achievements and future perspectives in whole-organ bioengineering Rocky Tuan. Timothy O’brien Stem Cell Res Ther 6:107
Ribeiro-Paes JT, Bilaqui A, Greco OT, Ruiz MA, Marcelino MY, Stessuk T, de Faria CA, Lago MR (2011) Unicentric study of cell therapy in chronic obstructive pulmonary disease/pulmonary emphysema. Int J COPD 6:63–71. https://doi.org/10.2147/COPD.S15292
Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BLM (2009) Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A 106:12771–12775. https://doi.org/10.1073/pnas.0906850106
Rosen C, Shezen E, Aronovich A, Klionsky YZ, Yaakov Y, Assayag M, Biton IE, Tal O, Shakhar G, Ben-Hur H, Shneider D, Vaknin Z, Sadan O, Evron S, Freud E, Shoseyov D, Wilschanski M, Berkman N, Fibbe WE, Hagin D, Hillel-Karniel C, Krentsis IM, Bachar-Lustig E, Reisner Y (2015) Preconditioning allows engraftment of mouse and human embryonic lung cells, enabling lung repair in mice. Nat Med 21:869–879. https://doi.org/10.1038/nm.3889
Schilders KAA, Eenjes E, van Riet S, Poot AA, Stamatialis D, Truckenmüller R, Hiemstra PS, Rottier RJ (2016) Regeneration of the lung: lung stem cells and the development of lung mimicking devices. Respir Res 17:1–16
Schneeberger K, Spee B, Costa P, Sachs N, Clevers H, Malda J (2017) Converging biofabrication and organoid technologies: the next frontier in hepatic and intestinal tissue engineering? Biofabrication 9. https://doi.org/10.1088/1758-5090/AA6121
Siddiqui FM, Diamond JM (2018) Lung transplantation for chronic obstructive pulmonary disease: Past, present, and future directions. Curr Opin Pulm Med 24:199–204
Soriano JB, Kendrick PJ, Paulson KR, Gupta V, Abrams EM, Adedoyin RA, Adhikari TB, Advani SM, Agrawal A, Ahmadian E, Alahdab F, Aljunid SM, Altirkawi KA, Alvis-Guzman N, Anber NH, Andrei CL, Anjomshoa M, Ansari F, Antó JM, Arabloo J, Athari SM, Athari SS, Awoke N, Badawi A, Banoub JAM, Bennett DA, Bensenor IM, Berfield KSS, Bernstein RS, Bhattacharyya K, Bijani A, Brauer M, Bukhman G, Butt ZA, Cámera LA, Car J, Carrero JJ, Carvalho F, Castañeda-Orjuela CA, Choi JYJ, Christopher DJ, Cohen AJ, Dandona L, Dandona R, Dang AK, Daryani A, de Courten B, Demeke FM, Demoz GT, De Neve JW, Desai R, Dharmaratne SD, Diaz D, Douiri A, Driscoll TR, Duken EE, Eftekhari A, Elkout H, Endries AY, Fadhil I, Faro A, Farzadfar F, Fernandes E, Filip I, Fischer F, Foroutan M, Garcia-Gordillo MA, Gebre AK, Gebremedhin KB, Gebremeskel GG, Gezae KE, Ghoshal AG, Gill PS, Gillum RF, Goudarzi H, Guo Y, Gupta R, Hailu GB, Hasanzadeh A, Hassen HY, Hay SI, Hoang CL, Hole MK, Horita N, Hosgood HD, Hostiuc M, Househ M, Ilesanmi OS, Ilic MD, Irvani SSN, Islam SMS, Jakovljevic M, Jamal AA, Jha RP, Jonas JB, Kabir Z, Kasaeian A, Kasahun GG, Kassa GM, Kefale AT, Kengne AP, Khader YS, Khafaie MA, Khan EA, Khan J, Khubchandani J, Kim YE, Kim YJ, Kisa S, Kisa A, Knibbs LD, Komaki H, Koul PA, Koyanagi A, Kumar GA, Lan Q, Lasrado S, Lauriola P, La Vecchia C, Le TT, Leigh J, Levi M, Li S, Lopez AD, Lotufo PA, Madotto F, Mahotra NB, Majdan M, Majeed A, Malekzadeh R, Mamun AA, Manafi N, Manafi F, Mantovani LG, Meharie BG, Meles HG, Meles GG, Menezes RG, Mestrovic T, Miller TR, Mini GK, Mirrakhimov EM, Moazen B, Mohammad KA, Mohammed S, Mohebi F, Mokdad AH, Molokhia M, Monasta L, Moradi M, Moradi G, Morawska L, Mousavi SM, Musa KI, Mustafa G, Naderi M, Naghavi M, Naik G, Nair S, Nangia V, Nansseu JR, Nazari J, Ndwandwe DE, Negoi RI, Nguyen TH, Nguyen CT, Nguyen HLT, Nixon MR, Ofori-Asenso R, Ogbo FA, Olagunju AT, Olagunju TO, Oren E, Ortiz JR, Owolabi MO, DNB MPA, Pakhale S, Pana A, Panda-Jonas S, Park EK, Pham HQ, Postma MJ, Pourjafar H, Poustchi H, Radfar A, Rafiei A, Rahim F, Rahman MHU, Rahman MA, Rawaf S, Rawaf DL, Rawal L, Reiner RC, Reitsma MB, Roever L, Ronfani L, Roro EM, Roshandel G, Rudd KE, Sabde YD, Sabour S, Saddik B, Safari S, Saleem K, Samy AM, Santric-Milicevic MM, Sao Jose BP, Sartorius B, Satpathy M, Savic M, Sawhney M, Sepanlou SG, Shaikh MA, Sheikh A, Shigematsu M, Shirkoohi R, Si S, Siabani S, Singh V, Singh JA, Soljak M, Somayaji R, Soofi M, Soyiri IN, Tefera YM, Temsah MH, Tesfay BE, Thakur JS, Toma AT, Tortajada-Girbés M, Tran KB, Tran BX, Tudor Car L, Ullah I, Vacante M, Valdez PR, van Boven JFM, Vasankari TJ, Veisani Y, Violante FS, Wagner GR, Westerman R, Wolfe CDA, Wondafrash DZ, Wondmieneh AB, Yonemoto N, Yoon SJ, Zaidi Z, Zamani M, Zar HJ, Zhang Y, Vos T, (2020) Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med 8:585–596. https://doi.org/10.1016/S2213-2600(20)30105-3
Stessuk T, Ruiz MA, Greco OT, Bilaqui A, de Ribeiro-Paes MJ, O, Ribeiro-Paes JT, (2013) Phase I clinical trial of cell therapy in patients with advanced chronic obstructive pulmonary disease: follow-up of up to 3 years. Rev Bras Hematol Hemoter 35:352–357. https://doi.org/10.5581/1516-8484.20130113
Strikoudis A, Cieślak A, Loffredo L, Chen YW, Patel N, Saqi A, Lederer DJ, Snoeck HW (2019) Modeling of fibrotic lung disease using 3D organoids derived from human pluripotent stem cells. Cell Rep 27:3709-3723.e5. https://doi.org/10.1016/j.celrep.2019.05.077
Sun Z, Li F, Zhou X, Chung KF, Wang W, Wang J (2018) Stem cell therapies for chronic obstructive pulmonary disease: current status of pre-clinical studies and clinical trials. J Thorac Dis 10:1084–1098
Tan Q, Choi KM, Sicard D, Tschumperlin DJ (2017) Human airway organoid engineering as a step toward lung regeneration and disease modeling. Biomaterials 113:118–132. https://doi.org/10.1016/j.biomaterials.2016.10.046
Tebyanian H, Karami A, Nourani MR, Motavallian E, Barkhordari A, Yazdanian M, Seifalian A (2019) Lung tissue engineering: an update. J Cell Physiol 234:19256–19270. https://doi.org/10.1002/jcp.28558
Tesei A, Zoli W, Arienti C, Storci G, Granato AM, Pasquinelli G, Valente S, Orrico C, Rosetti M, Vannini I, Dubini A, Dell’Amore D, Amadori D, Bonafè M (2009) Isolation of stem/progenitor cells from normal lung tissue of adult humans. Cell Prolif 42:298–308. https://doi.org/10.1111/j.1365-2184.2009.00594.x
Tindle C, Fuller M, Fonseca A, Taheri S, Ibeawuchi S-R, Beutler N, Katkar GD, Claire A, Castillo V, Hernandez M, Russo H, Duran J, Alexander LEC, Tipps A, Lin G, Thistlethwaite PA, Chattopadhyay R, Rogers TF, Sahoo D, Ghosh P, Das S (2021) Adult stem cell-derived complete lung organoid models emulate lung disease in COVID-19. bioRxiv Prepr Serv Biol. https://doi.org/10.1101/2020.10.17.344002
Tsuchiya T, Sivarapatna A, Rocco K, Nanashima A, Nagayasu T, Niklason LE (2014) Future prospects for tissue engineered lung transplantation: decellularization and recellularization-based whole lung regeneration. Organogenesis 10:196–207
Usui S, Shimizu T, Fujita K, Kishioka C, Sakakura Y (2000) Secretory cell differentiation and mucus secretion in cultures of human nasal epithelial cells: use of a monoclonal antibody to study human nasal mucin. Ann Otol Rhinol Laryngol 109:271–277. https://doi.org/10.1177/000348940010900307
Vargas-Valderrama A, Messina A, Mitjavila-Garcia MT, Guenou H (2020) The endothelium, a key actor in organ development and hPSC-derived organoid vascularization. J Biomed Sci 27:67
Vlahos R, Bozinovski S (2014) Recent advances in pre-clinical mouse models of COPD. Clin Sci 126:253–265
Weiner AI, Jackson SR, Zhao G, Quansah KK, Farshchian JN, Neupauer KM, Littauer EQ, Paris AJ, Liberti DC, Scott Worthen G, Morrisey EE, Vaughan AE (2019) Mesenchyme-free expansion and transplantation of adult alveolar progenitor cells: steps toward cell-based regenerative therapies. npj Regen Med 4. https://doi.org/10.1038/s41536-019-0080-9
Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP (2013) A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest 143:1590–1598. https://doi.org/10.1378/chest.12-2094
Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, Rogers AJ, Levitt J, Wiener-Kronish J, Bajwa EK, Leavitt A, McKenna D, Thompson BT, Matthay MA (2015) Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med 3:24–32. https://doi.org/10.1016/S2213-2600(14)70291-7
Yamamoto Y, Gotoh S, Korogi Y, Seki M, Konishi S, Ikeo S, Sone N, Nagasaki T, Matsumoto H, Muro S, Ito I, Hirai T, Kohno T, Suzuki Y, Mishima M (2017) Long-term expansion of alveolar stem cells derived from human iPS cells in organoids. Nat Methods 14:1097–1106. https://doi.org/10.1038/nmeth.4448
Zacharias WJ, Frank DB, Zepp JA, Morley MP, Alkhaleel FA, Kong J, Zhou S, Cantu E, Morrisey EE (2018) Type 2 alveolar cells are stem cells in adult lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 555:251–255. https://doi.org/10.1038/nature25786
Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, Deng K, Zhang L, Zou B, Cheng B, Xu J (2014) Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res 15. https://doi.org/10.1186/1465-9921-15-39
Zorn AM, Wells JM (2009) Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol 25:221–251. https://doi.org/10.1146/ANNUREV.CELLBIO.042308.113344
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Demchenko, A., Lavrov, A. & Smirnikhina, S. Lung organoids: current strategies for generation and transplantation. Cell Tissue Res 390, 317–333 (2022). https://doi.org/10.1007/s00441-022-03686-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-022-03686-x