Abstract
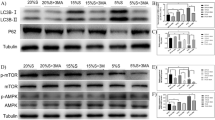
Fatty acids (FAs) play essential roles in regulating differentiation and proliferation by affecting gene expression in various cell types. However, their potential functions in bovine cells remain unclear. Herein, we examine the differentiation and proliferation of bovine skeletal muscle-derived satellite cells (MDSCs) after incubation with three types of representative FAs (palmitic acid, oleic acid and docosahexaenoic acid) by western blotting, immunofluorescence assays, flow cytometry analysis and EdU incorporation assays. The myotube fusion rate, myotube length and expression levels of muscle differentiation-related gene myogenin (MYOG) and myosin heavy chain 3 (MYH3) increased significantly, although the FAs did not affect proliferation. Additionally, FA-induced bovine MDSC differentiation increased ELOVL3 expression and relocation of ELOVL3 to cytoplasmic lipid droplets in the differentiation of bovine MDSCs. Moreover, the effect of FAs on bovine MDSC differentiation was inhibited upon ELOVL3 downregulation. Collectively, these data indicate that FAs promote bovine MDSC differentiation by regulating ELOVL3 expression.




Similar content being viewed by others
References
Allen RE, Luiten LS, Dodson MV (1985) Effect of insulin and linoleic acid on satellite cell differentiation. J Anim Sci 60:1571–1579
Bennett AM, Tonks NK (1997) Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science 278:1288–1291
Bosma M (2016) Lipid droplet dynamics in skeletal muscle. Exp Cell Res 340:80–186
Braun T, Gautel M (2011) Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat Rev Mol Cell Biol 12:349–361
Briolay A, Jaafar R, Nemoz G, Bessueille L (2013) Myogenic differentiation and lipid-raft composition of L6 skeletal muscle cells are modulated by PUFAs. Biochim Biophys Acta 1828:602–613
Cesar AS, Regitano LC, Poleti MD, Andrade SC, Tizioto PC, Oliveira PS, Felício AM, do Nascimento ML, Chaves AS, Lanna DP, Tullio RR, Nassu RT, Koltes JE, Fritz-Waters E, Mourão GB, Zerlotini-Neto A, Reecy JM, Coutinho LL (2016) Differences in the skeletal muscle transcriptome profile associated with extreme values of fatty acids content. BMC Genomics 17:961
Clarke SD (2000) Polyunsaturated fatty acid regulation of gene transcription: a mechanism to improve energy balance and insulin resistance. Br J Nutr 83:S59–S66
Deisenroth C, Itahana Y, Tollini L, Jin Y, Zhang Y (2011) p53-Inducible DHRS3 is an endoplasmic reticulum protein associated with lipid droplet accumulation. J Biol Chem 286:28343–28356
Denic V, Weissman JS (2007) A molecular caliper mechanism for determining very long-chain fatty acid length. Cell 130:663–677
Guo Z, Burguera B, Jensen MD (2000) Kinetics of intramuscular triglyceride fatty acids in exercising humans. J Appl Physiol 89:2057–2064
Han RH, Wang M, Fang X, Han X (2013) Simulation of triacylglycerol ion profiles: bioinformatics for interpretation of triacylglycerol biosynthesis. J Lipid Res 54:1023–1032
Houten SM, Wanders RJ (2010) A general introduction to the biochemistry of mitochondrial fatty acid beta-oxidation. J Inherit Metab Dis 33:469–477
Hurley MS, Flux C, Salter AM, Brameld JM (2006) Effects of fatty acids on skeletal muscle cell differentiation in vitro. Br J Nutr 95:623–630
Ichimura A, Hirasawa A, Hara T, Tsujimoto G (2009) Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat 89:82–88
Ji L, Gupta M, Feldman BJ (2016) Vitamin D regulates fatty acid composition in subcutaneous adipose tissue through Elovl3. Endocrinology 157:91–97
Jump DB (2009) Mammalian fatty acid elongases. Methods Mol Biol 579:375–389
Kanaley JA, Shadid S, Sheehan MT, Guo Z, Jensen MD (2009) Relationship between plasma free fatty acid, intramyocellular triglycerides and long-chain acylcarnitines in resting humans. J Physiol 587:5939–5950
Lee JH, Tachibana H, Morinaga Y, Fujimura Y, Yamada K (2009) Modulation of proliferation and differentiation of C2C12 skeletal muscle cells by fatty acids. Life Sci 84:415–420
Liu D, Xu JH, Tong HL, Li SF, Yan YQ (2017) Effect of ELOVL3 expression on bovine skeletal muscle-derived satellite cell differentiation. Biochem Biophys Res Commun S0006-291X:31476
Lu G, Morinelli TA, Meier KE, Rosenzweig SA, Egan BM (1996) Oleic acid-induced mitogenic signaling in vascular smooth muscle cells. A role for protein kinase C. Circ Res 79:611–618
Meex RC, Hoy AJ, Mason RM, Martin SD, McGee SM, Bruce CR, Watt MJ (2015) ATGL-mediated triglyceride turnover and the regulation of mitochondrial capacity in skeletal muscle. Am J Physiol Endocrinol Metab 308:E960–E970
Moon YA, Shah NA, Mohapatra S, Warrington JA, Horton JD (2001) Identification of mammalian long chain fatty acyl elongase regulated by sterol regulatory element-binding proteins. J Biol Chem 276:45358–45366
Nakamura MT, Nara TY (2003) Essential fatty acid synthesis and its regulation in mammals. Prostaglandins Leukot Essent Fatty Acids 68:145–150
Oh CS, Toke DA, Mandala S, Martin CE (1997) ELO2 and ELO3, homologues of the Saccharomyces cerevisiae ELO1 gene, function in fatty acid elongation and are required for sphingolipid formation. J Biol Chem 272:17376–17384
Ohno Y, Suto S, Yamanaka M, Mizutani Y, Mitsutake S, Igarashi Y, Sassa T, Kihara (2010) A ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc Natl Acad Sci U S A 107:18439–18444
Song J, Kwon N, Lee MH, Ko YG, Lee JH, Kim OY (2014) Association of serum phospholipid PUFAs with cardiometabolic risk: beneficial effect of DHA on the suppression of vascular proliferation/inflammation. Clin Biochem 47:361–368
Strable MS, Ntambi JM (2010) Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol 45:199–214
Sunaga H, Matsui H, Anjo S, Syamsunarno MR, Koitabashi N, Iso T, Matsuzaka T, Shimano H, Yokoyama T, Kurabayashi M (2016) Elongation of long-chain fatty acid family member 6 (Elovl6)-driven fatty acid metabolism regulates vascular smooth muscle cell phenotype through AMP-activated protein kinase/Krüppel-like factor 4 (AMPK/KLF4) signaling. J Am Heart Assoc 5:e004014
Tong HL, Yin HY, Zhang WW, Hu Q, Li SF, Yan YQ et al (2015) Transcriptional profiling of bovine muscle-derived satellite cells during differentiation in vitro by high throughput rna sequencing. Cell Mol Biol Lett 20(3):351–373
Tvrdik P, Westerberg R, Silve S, Asadi A, Jakobsson A, Cannon B, Loison G, Jacobsson A (2000) Role of a new mammalian gene family in the biosynthesis of very long chain fatty acids and sphingolipids. J Cell Biol 49:707–718
Zadravec D, Brolinson A, Fisher RM, Carneheim C, Csikasz RI, Bertrand-Michel J, Boren J, Guillou H, Rudling M, Jacobsson A (2010) Ablation of the very-long-chain fatty acid elongase ELOVL3 in mice leads to constrained lipid storage and resistance to diet-induced obesity. FASEB J 24:4366–4377
Funding
This work was supported by the breeding program for high-quality new varieties of genetically modified bovines from the National Major Transgenic Project [grant number 2014ZX08007-002].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The protocol utilised in this study to harvest cells from animal tissues was approved by the Animal Care Commission of the Northeast Agricultural University and Heilongjiang, P.R. China. Skeletal muscle tissues from newborn Chinese Simmental calves were obtained from the Shuangcheng abattoir, a local slaughterhouse in Heilongjiang, P.R. China.
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Xu, J., Liu, D., Yin, H. et al. Fatty acids promote bovine skeletal muscle satellite cell differentiation by regulating ELOVL3 expression. Cell Tissue Res 373, 499–508 (2018). https://doi.org/10.1007/s00441-018-2812-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2812-3